In just a short time, in many places, BTK inhibitors (BTKi) have largely replaced chemoimmunotherapy (CIT) for treatment naive CLL patients, while also playing an important role in relapsed/refractory CLL.
For a detailed list of the many BTK inhibitors in the pipeline, check out @AussieNeil’s post YABTKi (Yet Another BTKi): healthunlocked.com/cllsuppo...
For some of the most recent BTKi trial results, see these posts which also contain references to trials incorporating other therapies:
healthunlocked.com/cllsuppo...
healthunlocked.com/cllsuppo...
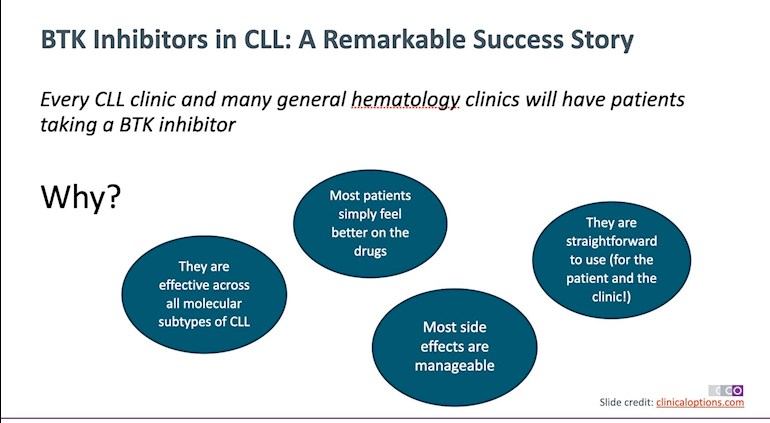
One of the great things about BTK inhibitors is that they “are effective across all molecular subtypes of CLL” and even provide a good treatment option for those patients who previously did poorly with CIT - patients with a 17p deletion or TP53 mutation.
Another advantage is that they are 'easy to administer and easy to take' and, on the whole, any 'side effects are mostly manageable', especially in an era of second and third generation BTK inhibitors. Supportive care medications, dose alterations and moving to an alternative BTKi can help to mitigate adverse reactions. A big bonus is that 'most patients start to feel much better when they’re taking a BTKi'.
A recent study has also shown that targeted therapies, including BTK Inhibitors can have a beneficial impact on pre-existing Auto-immune cytopenias associated with CLL.
healthunlocked.com/cllsuppo...
Since Ibrutinib first started to transform CLL treatment, a number of later, more targeted BTK inhibitors have been developed with Acalabrutinib and Zanubrutinib being the most well known.
Being more targeted, they have fewer off target side effects and have shown encouraging signs that they can reduce the risk of atrial fibrillation and hypertension which can affect some patients on Ibrutinib.
Later BTK inhibitors like Orelabrutinib and TG-1701 were reported on at the 2021 midyear conferences. So, too, has the new reversible BTK inhibitor, Pirtobrutinib, (LOXO-305) which has shown some great results for relapsed/refractory patients, including in the setting of Richter’s Transformation.
The downside of BTK inhibitors is that, when used as a monotherapy, they require ongoing, continuous therapy and this raises the toxicity risk and the risk of resistance.
In July 2022, an “International Consensus Statement on the Management of Cardiovascular Risk of Bruton's Tyrosine Kinase Inhibitors in CLL” A summary of those recommendations and a link to the full statement can be found here:
healthunlocked.com/cllsuppo...
In the not too distant future, trials will answer questions about:
- treatment sequencing
- the appropriateness of monotherapy Vs combination therapy for different patient groups
- the benefit or otherwise of adding a CD20 medication
- pausing, stopping, restarting therapy
- resistance mechanisms
- dose alterations
A recently published Phase III trial (ELEVATE - NCT02477696) compared Ibrutinib Vs Acalabrutinib in previously treated high risk CLL patients. After this year’s ASCO meeting, John C Byrd, MD, then professor of hematology at The Ohio State University (now at University of Cincinnati) said, “Acalabrutinib is a more selective inhibitor of [Bruton tyrosine kinase (BTK)] and has been known to have less adverse events (AEs) when compared across trials. [Until] today, no clinical trial has directly compared ibrutinib to acalabrutinib in previously treated CLL. Our trial presented here does this.”
cancernetwork.com/view/acal...
Another study, the ALPINE study, compares Ibrutinib vs Zanubrutinib in Patients With Relapsed/Refractory CLL. Interim analysis shows that zanubrutinib could improve clinical benefit vs ibrutinib.
Preliminary data from a Phase 2 study of Zanubrutinib in patients with B-cell malignancies intolerant to Ibrutinib/Acalabrutinib showed that zanubrutinib may provide a therapeutic option in patients intolerant to other BTK inhibitors.
Another very important trial is the CLL17 trial which compares Ibrutinib based therapy Vs Venetoclax based therapy Vs Ibrutinib + Venetoclax based therapy.
A summary of a recent presentation at the 16th International Conference on Malignant Lymphoma by Michael Hallek, Director of Germany’s Center for Integrated Oncology, presents a snapshot of the past, present and future of CLL continuous monotherapy vs fixed duration combination therapy.
'Therefore, one of the most important questions regarding CLL therapy is the comparison of two different treatment concepts, fixed duration therapy aimed at achieving maximal response (undetectable MRD) versus long-term disease control with single agent BTK inhibitors. The CLL17 protocol that has just opened recruitment will address this important question.'
healthunlocked.com/cllsuppo...
We’re also seeing some evidence that BTK inhibitors could be useful for patients who have progressed after Venetoclax treatment and that the Venetoclax treatment may be able to be continued again after a course of BTKi.
ashpublications.org/blood/a...
To get a good sense of the current state of play regarding BTK inhibitors and CLL, an excellent resource is provided by Clinical Care Options which produces resources designed for medical professionals.
The resources include a series of slidesets based on information presented at the recent EHA 2021 and ICML 2021 conferences. Topics covered include:
Evolving Strategies Using BTK Inhibitors in CLL: A Selective Approach to Improve Patient Outcomes
EHA/ICML 2021: The Current Role of BTK Inhibitors in CLL
EHA/ICML 2021: A Focus on Safety of BTK Inhibitors in the Management of CLL
EHA/ICML 2021: BTK Inhibitor-Based Combinations in Frontline CLL
A 2 hour webcast (FF is your friend!) is coming soon.
clinicaloptions.com/oncolog...
Clinical Care Options now has some 2022 resources about BTK inhibitors including this podcast:
clinicaloptions.com/oncolog...
It also has a great interactive decision making tool designed to help manage adverse events (AEs) associated with BTK inhibitors in haematological malignancies, including CLL.
clinicaloptions.com/oncolog...
You may need to register for access to the CCO site if you wish to view these resources.
An earlier article about managing toxicities associated with BTK Inhibitors appeared in the December 2020 Hematology ASH Education Program:
ashpublications.org/hematol...
For a review and summary about the different frontline treatments that might be considered for CLL in 2021, visit the following post:
healthunlocked.com/cllsuppo...
Previously pinned posts about BTK Inhibitors on this forum include:
Ibrutinib and the possibility of tumour flare / pseudo Richters Transformation when temporarily halting therapy:
healthunlocked.com/cllsuppo...
Ibrutinib cost
healthunlocked.com/cllsuppo...
Single-Agent Ibrutinib in Treatment-Naïve and Relapsed/Refractory Chronic Lymphocytic Leukemia: A 5-Year Experience (from 3 year’s ago)
healthunlocked.com/cllsuppo...
MUST READ for those on Ibrutinib/Imbruvica - concerning advice in pilot study on lower dosing - incl. danger of starting with a lower dose
healthunlocked.com/cllsuppo...
Hypertension & CV events on Ibrutinib
healthunlocked.com/cllsuppo...
Two references about Imbruvica (ibrutinib) and drug interactions
healthunlocked.com/cllsuppo....
High biotin doses may interfere with some SERUM blood tests, e.g. thyroid, anaemia. Those on Ibrutinib please note!
healthunlocked.com/cllsuppo...
UPDATE 27 October 2021:
BTK updates from the Lymphoma, Leukemia & Myeloma Congress 2021
Neil Kay, MD, Mayo Clinic, Rochester, MN, discusses
* MRD (measurable or minimal residual disease) in
* BTK combinations for CLL
* BTKi discontinuation in CLL - intolerance and disease progression
* Treating CLL intolerance or disease progression on BTKi
vjhemonc.com/video/v1ma7-h0...
These videos are also available on VJHemOnc's YouTube channel
UPDATE 31 October 2021:
The resistance mechanisms and treatment strategies of BTK inhibitors in B-cell lymphoma
onlinelibrary.wiley.com/doi...
UPDATE 11 November 2021: ‘This publication reviews the role of Bruton tyrosine kinase inhibitors, particularly when treating patients with agents that are to be taken continually and for an indefinite period of time, for the treatment of chronic lymphocytic leukemia/small lymphocytic leukemia, recapping key insights from a scientific interchange & workshop.’
onclive.com/view/updates-in...
(cdn.sanity.io/files/0vv8moc...
Image: From Clinical Care Options slideset - 'A Focus on Safety of BTK Inhibitors in the Management of CLL' clinicaloptions.com/oncolog...
UPDATE 18 November 2021: Targeting BTK in CLL: Expert Guidance on Selecting Single-Agent and Combination Therapy
This group of resources includes slide sets covering these topics:
- The Current Role of BTK inhibitors in CLL and
- Targeting BTK in CLL: Expert Guidance on Selecting Single-Agent and Combination Therapy
- An on demand webcast will be added soon
clinicaloptions.com/oncolog...
UPDATE 12 Dec 2021: Options for CLL patients who progress after BTK inhibitor therapy - ASH2021
youtu.be/vAMccDJKtjI or vjhemonc.com/video/vamccdjk...
The video is produced by VJHemOnc and shows an interview with Kerry Rogers, MD, The Ohio State University, Columbus, OH, at the recent ASH2021 conference. It is an excellent summary of the options that might be considered by a CLL patient who progresses on a BTK inhibitor.
In it, Dr Rogers discusses Venetoclax but highlights that it may not offer a durable response. Instead, she says, patients could consider clinical trial options for reversible BTK inhibitors, BTK degraders or drugs with a completely novel mechanism that are in development. She also suggests CAR-T treatment as a suitable alternative for some patients.
Knowing what mutations are associated with a patient’s disease progression is important. She explains that patients with a BTK (C481S) mutation might choose a reversible BTK inhibitor like pirtobrutinib (Loxo 305) but that those with a PCLG2 mutation would be better advised to consider options that don’t target BTK.
UPDATE 21 Dec 2021: 'BTK Inhibitors in the Presence of 17p Deletions or TP53 Mutation in CLL'
Susan M. O’Brien, MD in a recent video for CancerNetwork says: 'There’s 1 group [of patients for whom I] always use BTK inhibitors, and those would be the patients who have a 17p deletion or a TP53 mutation.’
'Clearly, patients with 17p deletion have remissions that appear to be lasting longer than they would with venetoclax. What we don’t know is [whether or not] ibrutinib is just a better drug than venetoclax for that subset or is finite therapy not a good idea in that subset. If I felt like there was a patient for [whom] I didn’t want to give a BTK inhibitor—and I’ve already stated that I can hardly think of what that setting would be—but if I did give them venetoclax-obinutuzumab, I’m not sure I would stop at 1 year. I might leave them on continuous therapy. But if I’m going to use the regimen as it’s FDA approved, then in that setting, I’m definitely going to go with my BTK inhibitors because the data are better.'
cancernetwork.com/view/btk-...
In a Clinical care Options article, Jennifer Woyach, MD explains this further:
‘Patients with high-risk disease, such as those with abnormal TP53, might be more strongly considered for BTK inhibitor–based therapy based on available data. Specific patient health factors can also potentially direct treatment. For example, someone who is receiving warfarin should not receive a BTK inhibitor and would be better suited for treatment with a BCL2 inhibitor–based regimen. Similarly, patients who have a history of hypertension that is difficult to control might not be optimal candidates for ibrutinib but may do very well with other BTK inhibitors or with venetoclax.’
clinicaloptions.com/oncolog...
UPDATE 16 Jan 2022: CLL12 Trial does not justify changing the current standard of “watch and wait": healthunlocked.com/cllsuppo...
UPDATE 19 Jan 2022: Choosing between a BTKi based therapy and a BCL2i based therapy for frontline CLL - healthunlocked.com/cllsuppo...
UPDATE 29 Jan 2022: BTKi sequencing and the role of 1st, 2nd and 3rd line BTKis youtu.be/S2xnjFkxY70
UPDATE 11 Feb 2022: See the following linked post by lankisterguy - a video of Dr Nicole Lamanna discussing some of the challenges associated with BTK inhibitors.
healthunlocked.com/cllsuppo...
UPDATE 25 Feb 2022: Understanding mechanisms of resistance to Pirtobrutinib in Chronic Lymphocytic Leukaemia healthunlocked.com/cllsuppo...
UPDATE 22 March 2022: The British Society of Haematology updated guideline document for the treatment of CLL: healthunlocked.com/cllsuppo...
(my emphasis)
*** PLEASE NOTE: This is an UNLOCKED reference post. If you wish to ask any questions, please do so in a separate post, not by replying here ***
How to write a post:
support.healthunlocked.com/...
Update 7 May 2022: A systematic review and meta-analysis has highlighted dermatologic toxicities associated with ibrutinib - healthunlocked.com/cllsuppo...
Update 12 May 2022: A short summary of the role of zanubrutinib in the treatment of CLL, highlighting its role for patients who take a Proton Pump inhibitor while requiring BTKi treatment:
onclive.com/view/dr-ma-on-u...
Also, a short summary about the ALPINE trial of zanubrutinib and ibrutinib -
onclive.com/view/dr-heyman-...
Post Updated 7 July 2022
 PS I don’t feel better after treatment began (Ibrutinib or Acalabrutinib) and the side effects aren’t mostly manageable for me.
PS I don’t feel better after treatment began (Ibrutinib or Acalabrutinib) and the side effects aren’t mostly manageable for me.