Many of us were shocked after our CLL/SLL diagnosis when we googled for survival times. Hopefully we subsequently read that search engine information from Google, Bing, etc., is way out of date, due to the fact that with CLL being a chronic disease, the relatively long survival times with this cancer first have to be lived through before updates are possible. As I've regularly replied, with BTKi drugs only being introduced in the last decade, we are still awaiting 10 year survival time updates. Given the USA's National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program seer.cancer.gov/ "is an authoritative source for cancer statistics in the United States.", I'm pleased to share the results of this paper; Evaluating population-level outcomes in Chronic Lymphocytic leukemia in the era of novel therapies using the SEER registry
pubmed.ncbi.nlm.nih.gov/385...
"In the last decade, novel agents such as BTK and BCL-2 inhibitors have revolutionized treatment of CLL/SLL, with clinical trials showing improved overall survival compared to chemotherapeutic agents. However, studies examining whether they have improved overall survival at the population level are lacking. We evaluated this by conducting a retrospective analysis of CLL/SLL patients registered in the National Cancer Institute's surveillance epidemiology and end results (SEER) database, analyzing overall survival (OS) in periods pre- and post-availability of novel agents, along with demographic information. Our results showed that median OS significantly improved over time [7.8 years (2000-2005), 9.1 years (2006-2013), and not reached (2014-2018) (p < 0.001)]"
Repeating those median overall survival times, with my emphasis;
7.8 years (2000-2005)
9.1 years (2006-2013)
Not reached (2014-2018)
Bear in mind that those recent statistics showing improvements, primarily include people who had subsequent treatments with a BTK or BCL-2 inhibitor after prior treatment(s) with older, chemoimmunotherapy treatments. Those older chemoimmunotherapy treatments, typically Bendamustine + Rituximab (BR) or Fludarabine + Cyclophosphamide + Rituximab (FCR), selected for tougher to treat sub-clones, which they cause through inducing DNA damage that will hopefully trigger apoptosis if the TP53 gene isn't mutated or missing (17p del). They destroy CLL cells via DNA damage that inhibitor drugs can't cause, because these revolutionary drugs don't work by disrupting CLL cell division but by inhibiting cell messaging that normally triggers apoptosis when a B cell is no longer needed. (Note that it is the alkylating agents, the 'chemo' in the chemoimmunotherapy combination that does the DNA damage. Rituximab is the anti-CD20 monoclonal antibody (immunotherapy) component that targets the CD20 on our CLL and other maturing B cells for destruction by our immune system.)
This news gets even better, as illustrated by this study of 309 Italian patients prescribed ibrutinib, the first FDA approved BTKi for CLL.
Real-World Outcome of Treatment with Single-Agent Ibrutinib in Italian Patients with Chronic Lymphocytic Leukemia: Final Results of the EVIdeNCE Study (with my emphasis)
pubmed.ncbi.nlm.nih.gov/385...
After a median follow-up of 23.9 months, 29.8% of patients discontinued ibrutinib (1L: 24.6%, 2L: 29.9%, ≥3L: 39.1%), mainly owing to adverse events (AEs)/toxicity (14.2%). The most common AEs leading to discontinuation were infections (1L, ≥3L) and cardiac events (2L). The 2-year retention rate was 70.2% in the whole cohort (1L: 75.4%, 2L: 70.1%, ≥3L: 60.9%). The 2-year PFS and OS were, respectively, 85.4% and 91.7% in 1L, 80.0% and 86.2% in 2L, and 70.1% and 80.0% in ≥3L. Cardiovascular conditions did not impact patients' clinical outcomes. The most common AEs were infections (30.7%), bleeding (12.9%), fatigue (10.0%), and neutropenia (9.7%), while grade 3-4 atrial fibrillation occurred in 3.9% of patients. No new safety signals were detected. These results strongly support ibrutinib as a valuable treatment option for CLL.
So this paper confirms that ibrutinib, which began the inhibitor treatment revolution, had a concerningly high discontinuation rate, "mainly owing to adverse events (AEs)/toxicity". The even better news is that we now have second and third generation FDA approved BTKis, which have significantly lower adverse event and toxicity profiles - acalabrutinib, pirtobrutinib and zanubrutinib, with more BTKis and BCL-2is to come. See: healthunlocked.com/cllsuppo...
As this recent post by CLLerinOz notes, with my emphasis; It's time to start talking about ways to enhance survivorship for those with chronic lymphocytic leukemia
healthunlocked.com/cllsuppo...
"In recent years, survivorship strategies that aim to support and enhance the post treatment lives of cancer patients have been a prominent feature of the care of those with metastatic cancer in particular. At the same time, highly effective and less toxic therapies have been leading to a significant reduction in CLL-related mortality, increasing the need for a similar approach to be taken with people who are now surviving with CLL in ever increasing numbers."
Our Pinned Post section healthunlocked.com/cllsuppo... is full of survivorship strategies posts that will help you surpass those reported median survival times, for example:-
30 Tips for living well with CLL
healthunlocked.com/cllsuppo...
Vaccinations - keep up to date with vaccinations/boosters to reduce the severity of any potentially life threatening infections
healthunlocked.com/cllsuppo...
Learn more about your immune system
healthunlocked.com/cllsuppo...
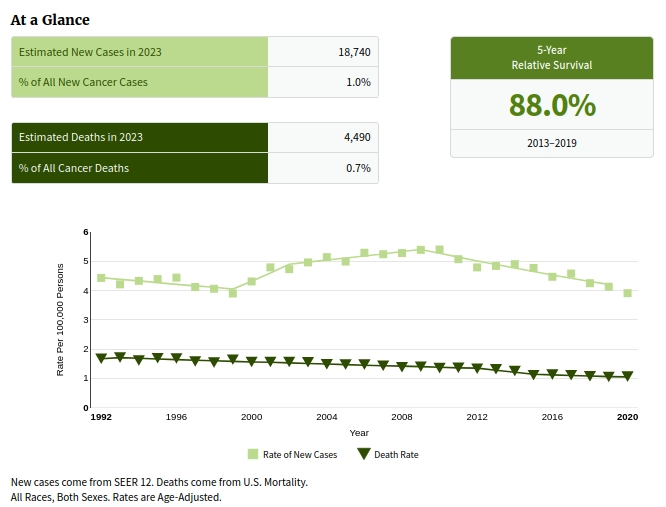
Graphic is from CLL/SLL SEER data, showing from 1992 to 2020, plots of the rate of new cases (4.4 to 3.9 per 100,000) and death rates (1.7 to 1.1 per 100,000)
Neil