The latest edition of the journal Blood has two articles that highlight the issue of cardiotoxicity in patients treated with acalabrutinib.
The cardiotoxicity of ibrutinib has been well recorded but the situation with the newer BTK inhibitor, acalabrutinib, has been less clear.
In the first article, "Ventricular arrhythmias and sudden death events following acalabrutinib initiation", Seema A. Bhat et al show that:
"Patients treated with next generation BTKi therapy acalabrutinib still see a >8 fold risk of ventricular arrhythmia and sudden death events.
Ventricular arrhythmias may be a class effect of BTKi therapy, and vigilance is needed."
ashpublications.org/blood/a...
The second of these articles, titled "Cardiotoxicity in patients treated with acalabrutinib" by Petra Langerbeins states:
"Although the observed cardiotoxic adverse events in nearly 3% of patients treated with acalabrutinib was lower than that reported in patients treated with ibrutinib, the percentage was eightfold higher than that in similar untreated control patients. These data indicate that VAs may be a class-specific effect of Bruton tyrosine kinase inhibitors (BTKi’s)."
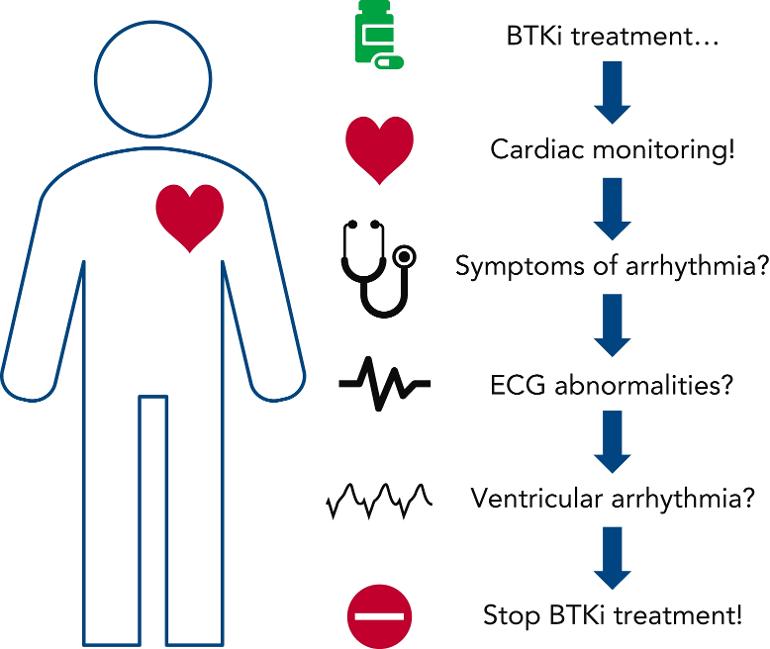
"Bhat et al provide the first evidence of an increased risk for VAs in patients treated with the second-generation BTKi acalabrutinib. Although the frequency of this severe cardiotoxicity was less common than with ibrutinib, the data strongly indicate that there is a cardiotoxicity class effect with BTKi therapies. In clinical practice, this should translate into systematic cardiac monitoring of all patients treated with BTKi’s, with careful assessment of cardiovascular risk factors and a baseline electrocardiogram before treatment is initiated. During treatment with BTKi’s, clinicians should inquire about symptoms of VA (lightheadedness, palpitations, or syncope) and perform diagnostic electrocardiograms with a low threshold (see figure). Other targeted treatment modalities may be available, so patients who develop cardiotoxicity associated with BTKi’s may be advised to discontinue treatment with them and switch to an alternative treatment regimen."
Those with a risk of cardiotoxicity could consider a time-limited treatment to reduce the possibility of long-term toxicities (Bhat et al reported a median time to ventricular arrhythmia (VA) of more than 1 year). She also recommends clinical trials be developed to assess long-term cardiotoxicity.
"In conclusion, it is vital that patients and caregivers be aware of the severe cardiotoxicity associated with ibrutinib and also with the second-generation BTKi acalabrutinib. Consideration of cardiovascular adverse effects is an important decision-making tool for selecting treatment for CLL, even in a regimen based on acalabrutinib."
Note that, while this event was recorded at a rate eightfold higher than that recorded in similar untreated control patients, it was observed in just 3% of patients treated with acalabrutinib. It's a call for careful screening and monitoring, rather than a call for avoiding acalabrutinib. It's also a reminder to those whose screening identifies that they may be at risk to consider time-limited therapy while others who select acalabrutinib over longer periods should maintain vigilance and be monitored carefully throughout their treatment.
(my emphasis)
1. Seema A. Bhat, John Gambril, Leylah Azali, Sunnia T. Chen, Lindsay Rosen, Marilly Palettas, Tracy E. Wiczer, Sujay Kalathoor, Qiuhong Zhao, Kerry A. Rogers, Adam Kittai, Michael Grever, Farrukh Awan, Patrick Ruz, John C. Byrd, Jennifer Woyach, Daniel Addison; Ventricular arrhythmias and sudden death events following acalabrutinib initiation. Blood 2022; 140 (20): 2142–2145. doi: doi.org/10.1182/blood.20220...
2. Petra Langerbeins; Cardiotoxicity in patients treated with acalabrutinib. Blood 2022; 140 (20): 2096–2097. doi: doi.org/10.1182/blood.20220...