An update of the long-term follow-up results from the original 300-patient FCR (fludarabine, cyclophosphamide, and rituximab) study initiated at MD Anderson in 1999, Sustained remissions in CLL after frontline FCR treatment with very-long-term follow-up, reports ~47% of patients with mutated IGHV have achieved remissions approaching 24 years. ashpublications.org/blood/a... Basically, if you have mutated IGHV (IGHV-M) and you achieve a 10 year remission, you have a very good chance of never needing further treatment for your CLL. "Only 4 of 45 patients (9%) with IGHV-M progressed beyond 10 years."
Note:
IGHV-M = mutated IGHV
IGHV-UM = unmutated IGHV
PFS = progression free survival
frontline = first treatment
In the associated commentary by Matthew Davids MD, Functional cure reported in CLL, ashpublications.org/blood/a...
"..for young, fit patients with CLL, we need to consider their outcomes not only at 5 years after starting therapy but also at 20 years and beyond. With a median follow-up of 19 years, they report a median PFS for patients with IGHV-M CLL of 14.6 years. Disease progression beyond 10 years was uncommon, suggesting that some patients had “functional cure” of their CLL; however, a 6.3% cumulative risk of therapy-related myelodysplastic syndrome (MDS)/acute myeloid leukemia (AML) was observed."
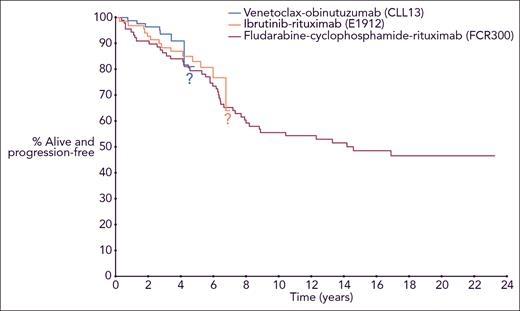
The big advantages of combination targeted therapies (venetoclax + obinutuzumab and ibrutinib+ rituximab shown in the accompanying PFS plots), are that not only are they proving superior to FCR, you don't need to be a young (aged 65 or younger), fit patient to go through these treatments, there is no longer a significant PFS difference between IGHV-M and IGHV-UM and there isn't the cumulative risk (6.3%) of developing therapy-related myeloid neoplasms (tMNs), i.e. MDS and AML. Of note, these were fatal in 16 of 19 (84%) of the cases.
Dr David comments "Although we are optimistic about the durability of remissions achieved with long-term sequential use of targeted therapies, our answer to that important question today is that we do not know, because follow-up with the targeted therapies is not nearly long enough." This is why the survival statistics you'll find through an online search are so misleading. We don't yet have 10 year survival statistics for the improved combination targeted therapy treatments, because clinical trials for these only commenced within the last 7 years. Hence the question marks in the above PFS plots. Importantly, the median age at diagnosis for CLL is about 70 and the median age for first treatment about 75.
Prior to the advent of targeted therapies, we only had chemoimmunotherapy treatments, typically BR (bendamustine+rituximab) or a very old drug chlorambucil to offer to most patients needing treatment. FCR was the first treatment which was proven to increase life expectancy, but very few patients could benefit from it. According to the US SEER database, seer.cancer.gov/statfacts/h... around 32% of CLL diagnoses occur at or before age 64. The IGHV-M : IGHV-UM ratio is about 50:50 at diagnosis, but because IGHV-M is associated with a longer time to first treatment, more IGHV-UM than IGHV-UM folk need treatment; the ~30% of patients who never need treatment are predominantly IGHV-M.
Dr Davids concludes, "So now when I sit with a young patient with CLL and he or she asks what I would choose for frontline therapy, the answer is less clear than it used to be. Based on the very-long–term data from Thompson et al, FCR remains a reasonable choice for young, fit patients with IGHV-M CLL, particularly in countries where access to frontline targeted therapies is limited. But given the risks of secondary MDS/AML, prolonged myelosuppression, and infectious complications, my usual answer now is that I would choose a targeted therapy regimen and hope that we will continue to develop new approaches to add to the impressive array of novel therapies we already have available for patients with CLL. If past performance is a predictor of future results in CLL, then the progress of the last decade bodes well for the therapeutic advances we are likely to continue to make in the next decade and beyond."
(My emphasis)
Neil