Published 4 months ago, journals.lww.com/hemasphere... is an update of the Phase 2 study S149, initiated in 2017 and previously reported here in "Novel Combos Are Shaking Up the CLL Landscape but FCR still has its place" healthunlocked.com/cllsuppo...
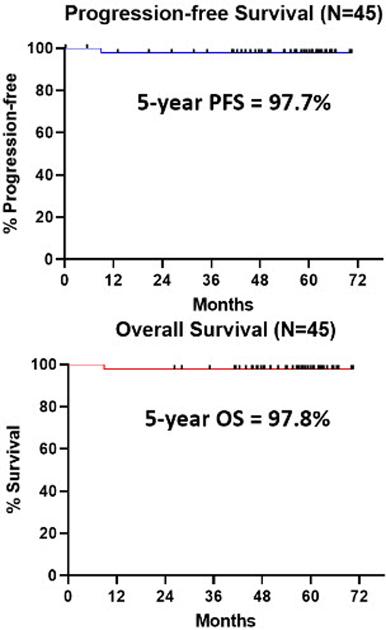
The update reports outcomes with a median follow-up of 56.8 months. The PFS and OS are, as depicted, very high, even allowing for favourable age profile (25-71, median 60) and CLL genetics (100 % IGHV mutated, 69% del 13q). The PFS and OS curves reflect one patient death (from heart failure).
Summary/Conclusion: The iFCG regimen, using only 3 cycles of chemotherapy (as opposed to 6 cycles of chemoimmunotherapy) achieves a very high rate of U-MRD in previously-untreated pts with CLL with IGHV-M CLL. No pt had disease progression with a median follow-up of close to 5 years. The 5-year PFS is 97.7%; this is favorable compared to 5-year PFS of ≈65% with FCR (CLL10), ≈70% with ibrutinib (A041202 trial), and 81% with ibrutinib (RESONATE-2) for IGHV-M CLL. Not unexpectedly, MRD recurrence during follow-up correlated with MRD positivity by NGS during therapy.
I have no doubt that these results are easily good enough to justify a phase 3 study enrolling many more patients. Will it happen? I guess it depends on whether potential sponsors see something to be gained, or lost. A phase 3 study would certainly burn a heap of cash. A "good" result could widen the market, or more likely could see a flagship product nibbling away at its own market share ("Stop taking Ibrutinib after a year??? after our lawyers worked their butts off to get the patents stretched out into the 2030s?"). Anyway, who wants to be associated with old-hat chemo drugs? Well, maybe some CLL patients do.