This 29 November 2022 review article published in the Blood Cancer Journal presents an up-to-date treatment algorithm for CLL.
nature.com/articles/s41408-...
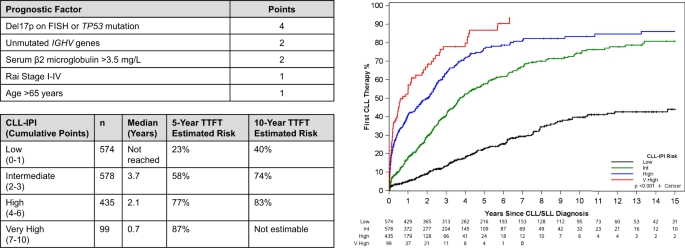
"The treatment landscape for patients with chronic lymphocytic leukemia (CLL) has changed considerably with the introduction of very effective oral targeted therapies (such as Bruton tyrosine kinase inhibitors and venetoclax) and next-generation anti-CD20 monoclonal antibodies (such as obinutuzumab). These agents lead to improved outcomes in patients with CLL, even among those with high-risk features, such as del17p13 or TP53 mutation and unmutated immunoglobulin heavy chain (IGHV) genes. Selecting the right treatment for the right patient requires consideration of disease characteristics and prior treatment sequence, as well as patient preferences and comorbidities. The CLL-International Prognostic Index (CLL-IPI) remains the best-validated tool in predicting the time to first therapy among previously untreated patients, which guides selection for early intervention efforts. This review summarizes our current approach to the management of CLL, right from the time of diagnosis through relapsed disease."
Things that stand out:
1. Only those patients who meet the 2018 iwCLL criteria for initiation of therapy should be offered treatment . In the Relapsed/Refractory setting, progressive disease often does not immediately equate to an indication for starting a treatment or changing the current treatment, until patients meet the 2018 iwCLL criteria for therapy.
2. A clinical trial should be considered for all patients who meet the 2018 iwCLL guidelines for the commencement of therapy, regardless of their risk classification and regardless of whether they are considering their first or subsequent therapy.
3. Venetoclax- and BTKi-based treatments are both excellent options for patients without a TP53 aberration and either is favoured over CIT in this setting, regardless of IGHV mutation status. (See healthunlocked.com/cllsuppo... for more information about how IGHV might influence treatment choice).
When a BTK inhibitor is selected, either acalabrutinib or zanubrutinib is preferred in most patients.
See the article for more details about each of these treatments, including guidance about when one might be more suitable than another for particular patients.
4. Continuous BTK inhibitor-based treatment in patients with TP53 disruption may provide superior PFS in the frontline setting.
5. Patient fitness is another important consideration. Very frail patients who score 'unfit' with a CIRS score of ≥ 6-8 and whose comorbidities preclude BTKi or venetoclax, might consider single-agent obinutuzumab in addition to those therapies already referenced above.
6. "MRD at the end of CLL therapy (and potentially also as a dynamic assessment) remains a powerful prognostic tool in the novel agent era, particularly with time-limited venetoclax-based approaches. Achieving uMRD with continuous BTKi treatment, in contrast, does not impact PFS."
7. "Patients with relapsed CLL should undergo a comprehensive assessment of their disease status, including bone marrow aspirate and biopsy and typically CT imaging (chest/abdomen/pelvis). A positron emission tomography (PET)/CT scan is preferred if there is suspicion for Richter transformation, and biopsy of lesions with a maximum standardized uptake value (SUVmax) ≥ 5 should be strongly considered (sensitivity 96%, specificity 21%, negative predictive value 86%, among patients experiencing disease progression on BTKi). TP53 mutation testing and CLL FISH panel should be repeated. Evaluating patients with relapsed CLL also requires the same considerations for medical comorbidities and frailty. The added calculus comes with review of prior treatment regimens and outcomes"
See the full article for details about the various treatment alternatives, depending on which frontline therapy a patient has already received and depending on whether the patient is changing therapy because of toxicity or disease progression.
8. "A disease flare phenomenon, characterized by rapidly progressive symptoms and adenopathy and rarely histopathologic evidence of Richter transformation, may occur during interruptions in BTKi treatment, but particularly after stopping the covalent BTKi until the next line of therapy is started. We recommend continuing the BTKi during the transition period to next-line therapy, particularly through venetoclax ramp-up until the target dose is reached ."
9. Cell therapy evaluation is recommended during remission on the second targeted agent.
The article discusses different cell therapies, including Allogeneic HSCT, CAR-T and CAR-NK therapies.
It also raises the need to consider any complications a patient might be experiencing, such as autoimmune cytopenias, which occur in ~5–10% of CLL patients, and other autoimmune complications.
"Richter transformation remains a feared complication of CLL in the novel agent era, retaining a poor prognosis." The article directs readers to recently published papers about Richter transformation of CLL.
CONCLUSION:
"Momentous gains in efficacy and tolerability of targeted therapies have continued to shape a dynamic treatment landscape, increasing the ability to individualize treatment to the goals and values of the patient. Pressing questions in the next phase of CLL research include how best to combine novel agents, the sequencing of these treatments, and administering time-limited treatments to achieve deep remissions that allow stopping therapy."
Hampel, P.J., Parikh, S.A. Chronic lymphocytic leukemia treatment algorithm 2022. Blood Cancer J. 12, 161 (2022). doi.org/10.1038/s41408-022-...
Note: not all of the therapies recommended in this algorithm are available to patients in all places. In some places, outside of a clinical trial, the only initial therapy available to patients without a TP53 mutation/17p deletion is chemo/chemoimmunotherapy. This makes the recommendation for all patients to consider a clinical trial, whatever their stage of CLL, all the more important.