Well, looks like at least one desiccated thyroid "supplement" does contain some thyroid hormone. But I am not entirely happy with this paper.
The second paper is an analysis of several over-the-counter supplements.
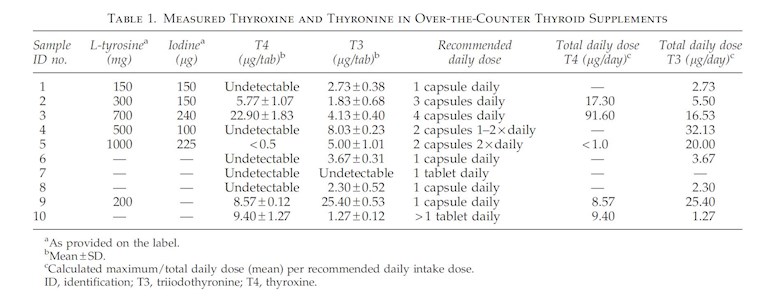
The image at the top of this post is taken from that paper.
J Clin Pharm Ther. 2019 Jun 18. doi: 10.1111/jcpt.12996. [Epub ahead of print]
A 'Natural' thyroid storm!
Mohebbi MR1, Chen AT2.
Author information
1 Department of Emergency Medicine, St Joseph's Medical Center, Stockton, California.
2 Department of Physiology and Pharmacology, University of the Pacific, Stockton, California.
Abstract
WHAT IS KNOWN AND OBJECTIVE:
Over the counter supplements are often taken for granted during medication reconciliation in the emergency department. Supplements are not regulated by FDA, and some can be potentially dangerous.
CASE SUMMARY:
We report a case of thyrotoxicosis secondary to over the counter bovine thyroid supplements. Our patient presented with atrial fibrillation with rapid ventricular response refractory to calcium channel blockers. Had we not known about the supplement, the course of treatment would have been different with potential adverse outcome.
WHAT IS NEW AND CONCLUSION:
Natural thyroid supplements are marketed as over the counter products and are largely unregulated. Thyroid extracts have been found to have disparaging inconsistencies in composition, delivering anywhere from non-existent to supratherapeutic doses. Thyroid supplements should be regulated considering the potential side effects.
© 2019 John Wiley & Sons Ltd.
KEYWORDS:
OTC; cardiovascular disease; clinical pharmacokinetics; clinicians; over-the-counter medications; supplement; thyroid
PMID: 31211437
DOI: 10.1111/jcpt.12996
ncbi.nlm.nih.gov/pubmed/312...
Thyroid Dysfunction: Hypothyroidism, Thyrotoxicosis, and Thyroid Function Tests normal
Thyroxine and Triiodothyronine Content in Commercially Available Thyroid Health Supplements
Grace Y. Kang, Jonathan R. Parks, Bader Fileta, Audrey Chang, Maged M. Abdel-Rahim, Henry B. Burch, and Victor J. Bernet
Published Online: 25 Sep 2013 doi.org/10.1089/thy.2013.0101
Abstract
Background: As defined by the Dietary Supplement Health and Education Act 1997, such substances as herbs and dietary supplements fall under general Food and Drug Administration supervision but have not been closely regulated to date. We examined the thyroid hormone content in readily available dietary health supplements marketed for “thyroid support.”
Methods: Ten commercially available thyroid dietary supplements were purchased. Thyroid supplements were dissolved in 10 mL of acetonitrile and water with 0.1% trifloroacetic acid and analyzed using high-performance liquid chromatography for the presence of both thyroxine (T4) and triiodothyronine (T3) using levothyroxine and liothyronine as a positive controls and standards.
Results: The amount of T4 and T3 was measured separately for each supplement sample. Nine out of 10 supplements revealed a detectable amount of T3 (1.3–25.4 μg/tablet) and 5 of 10 contained T4 (5.77–22.9 μg/tablet). Taken at the recommended dose, 5 supplements delivered T3 quantities of greater than 10 μg/day, and 4 delivered T4 quantities ranging from 8.57 to 91.6 μg/day.
Conclusions: The majority of dietary thyroid supplements studied contained clinically relevant amounts of T4 and T3, some of which exceeded common treatment doses for hypothyroidism. These amounts of thyroid hormone, found in easily accessible dietary supplements, potentially expose patients to the risk of alterations in thyroid levels even to the point of developing iatrogenic thyrotoxicosis. The current study results emphasize the importance of patient and provider education regarding the use of dietary supplements and highlight the need for greater regulation of these products, which hold potential danger to public health.