SoonerMark, Istomin, and any other patients interested in the Artificial Urinary Sphincter, aka AMS800, as manufactured by American Medical Systems, Inc. and supplied by Boston Scientific. Here is a synopsis of my own experience.
Preface to the following is that I also have indolent CLL and am fortunate to have never had any classic symptoms or been treated. Other than in-the-toilet immunoglobulins, my labs have improved over time and now inexplicably hang around normal levels across the board.
My early PCa history involved nerve-sparing RALP surgery in 2013. The cancer was not organ-confined, with 2 smallish surgical margins, extra-cap involvement, and invasion of my right seminal vesicle. Final biopsy staged at pT3a/3b pNx, with 30 % of the prostate cancerous and a Glason of 4+3. Five lymph nodes biopsied at the start of the RALP were positive for CLL (as expected), but all negative for PCa.
A few days before my initial post-surgery consultation with my surgeon, I found the online document titled, Adjuvant and Salvage Radiotherapy after Prostatectomy: ASTRO/AUA Guideline, circa 2013. That document has had two major revisions since then, but having reviewed the rationale for IMRT for each of the four major pathology negatives in the 2013 document (1. non-organ confined, 2. extra-capsular involvement, 3. seminal vesicle involvement, & 4. positive lymph nodes), I commented off the top to my surgeon that, "It looks to me like any one of these three negatives would be a good reason to consider RT, and with three of the four, it seems like a no-brainer". He reply was, "I wouldn't disagree". So, at my insistence, we went forward with 8 weeks of IMRT at about 3 months post-RALP, rather than the 6 months recommended for continence improvement. I said then, and would repeat now, that I was willing to risk the prospect of improved continence in the interest of hitting any remaining cancer earlier. (My incontinence was an issue before the IMRT and, based on a comment made the day after surgery about damage to my urethra during the placement of the catheter, I now assume I would not likely have improved to a satisfactory level of continence even if I had waited the recommended six months for RT.)
IMRT bought me about 3 1/2 years of undetectable PSA, with the first two post-RALP labs done locally at 6 week and 12 weeks. PSA for both was 0.03. Not a bad result considering the advance state of the final pathology. Biochemical recurrence came back with radical doubling time (45-60 days). I had two sets of low-res bone/CT scans during the PSA ramp, with none showing signs of metastatic disease. I also had genetic testing as part of a clinical trial for about 120 defects and surprisingly found none. In the fall of 2016 with a PSA of 25+ I did one 3-month round of ADT. My response was excellent and I have been off treatment with undetectable PSA (I recently achieved a new post-RALP nadir of 0.02) and normal range T since. In this regard, I am an outlier PCa n=1 patient for sure, and a very happy one at that.
I had my urodynamic evaluation and, after reviewing the universally positive responses by patients, made the decision to go forward with the AUS surgery in the middle of 2016. That was just prior to going on ADT. That implant worked flawlessly until it failed in the spring of this year. Due to the pandemic, I've had to wait until 10 days ago for the replacement. As with all AUS surgeries, I now get to wait out the 6 weeks healing period before activation.
That's my historical overview and what follows is my more personal experience with the surgery, recovery, period, and general commentary on the device.
First thing to do (if you haven't already done it), is to review the information at the mfg's website linked at the end of this post. And while it is now 8 years old, I also recommend reviewing the research paper that compares the various male incontinence interventions including the AUS. As stated in that paper and elsewhere, the AMS800 is considered the "Gold Standard" for male urinary incontinence. After my AUS #1 experience, I wholeheartedly agree with that characterization. Depending on how bad your incontinence is, the less invasive male sling options may in fact work for you. I know a PCa patient in my town who had that procedure and is completely satisfied with the results. As I understand that device, it basically repositions the bladder and seems to involve some degree of surgeon skill (a very good thing for any surgery) and a measure of random luck in achieving the promised effectiveness. (This procedure and the AMS 800 are both described in the video linked at the end.)
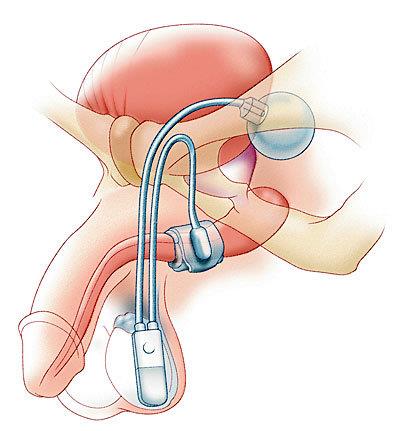
Understand up front that the surgery is much more invasive than a robotic prostatectomy. Two incisions are required, one down low on your abdomen on the preferred activation side, and the killer cut that basically runs from the back of you scrotum to the edge of you anus. That's a big OUCH! compared to the one small incision and the several holes for a RALP. But the most disconcerting after-effect is the abuse to your "privates". A least some of that is the result of wound irrigation used to clean the scrotum and reduce risk of infection. The device has three major components connected by non-kink plastic tubing. I like to think of it as similar to a hydraulic brake system, with the operation happening in reverse. The cuff goes around the urethra, the activation module in the scrotum side of preference (usually based on the patient being right- or left-handed), and the reservoir located low down on the abdomen on the same side as the activator. There are several solutions that are used for the fluid. My doc uses an agent with a contrast solution, so leakage will be visible on Xrays. Unless the patient is allergic to them, the device components are coated with a slow-release antimicrobial coating called InhibtZone (rifampin and minocycline HCl). You will also be on oral antibiotics for 10 days or so.
Risks for the device are possible infection, mechanical failure, fluid leakage, or physical damage due to accident or physical blow to one of the components. Activities that would put pressure on the cuff, such as bike and horse riding, are discouraged. Also note the warning in the Boston Scientific PDF file about device non-critical heating during extended MRI scans.
Post-surgery, sitting for the first week is a delicate matter. I recommend some sort of u-shaped foam cushion to relieve pressure on the larger incision and genitalia until they heal. That incision is glued, so no bandage is required. A small waterproof bandage is used for the abdominal incision. As with all surgeries, I recommend rejecting the opioids altogether and, if possible, managing the post-surgery pain/discomfort with Tylenol alone. As everyone's pain threshold is different, that may or may not work for others. The main reason I try to get off/avoid opioid pain meds is to reduce/eliminate the risk of constipation caused by them. IMO, stool softeners don't help very much with opioid-caused constipation. With this also, mileage may vary. As with most abdominal surgeries, you will be requested to not lift anything over 10 pounds for 6 weeks. Good luck with that, but I do always take it easy for the first three weeks or so.
As for the impact of the AUS on your QOL? . . . Well, if you have bad enough incontinence to be considered for the surgery, your life will most likely be transformed. With AUS #1 (which due to my RT, was sized a bit on the large side), I switched over from Guards to Shields and only several times a month did I usually need to use more than one per day. Some days, other than the dribbles that urinary impatience causes, pads were basically dry. I rarely ever had a leakage event that caused a wet spot on trousers and I estimated my over all continence was about 95%. (with a somewhat oversized cuff.) Stress incontinence is still a possibility, but in my case so minor as to usually be inconsequential.
In summary, I have just had cataract surgery on both eyes and that is the only medical intervention that has even come close to the QOL improvement that I got from the AUS. I found a patient support blog/forum thread when I was considering mine. Not a single patient that had received the AUS, including those who for one reason or another had to have it removed, said that they regretted or would not make the decision to have the AUS surgery again. I'm in the same camp and can't speak strongly enough that the benefits far outweigh any short and long-term risks involved. Admittedly, it is a bit creepy to wake up from surgery and find your genitalia literally black & blue and foreign objects scattered about the most private portions of your body. However, once it's activated, and you have mastered the technique involved in getting a grip on the slippery activation module, you will literally be able to "pee at the press of a button". (Actually it's a flexible bulb on the end of a device that looks and feels a bit like a 3/4 size thumb drive, but you get the idea.)
Good luck to both you guys and anyone else reading this who might be considering the AUS. Please feel free to ask questions here or, if you prefer, direct message them to me.
In the meantime, "Go for Gold" and Try to Stay Safe & Be Well - Captain K9, aka cujoe
Resources
Male Stress Urinary Incontinence: A Review of Surgical Treatment Options and Outcomes – circa 2012:
hindawi.com/journals/au/201...
The following links from the Boston Scientific web site should be very helpful:
Video on male incontinence that discusses male sling and AUS:
youtube.com/watch?v=1xNnISi...
Boston Scientific AMS 800 “Instructions For Use” covering most aspects of the device and including some clinical study data on effectiveness and causes of failure:
bostonscientific.com/conten...
Boston Scientific Physician’s Info Page with Physician’s Brochure, AMS activation animation video, and Surgical Prep Instructions: (Be sure to view them all.)
bostonscientific.com/en-US/...
And if you want to dig through the most recent clinical results on the AMS 800, you can find most of those studies here: