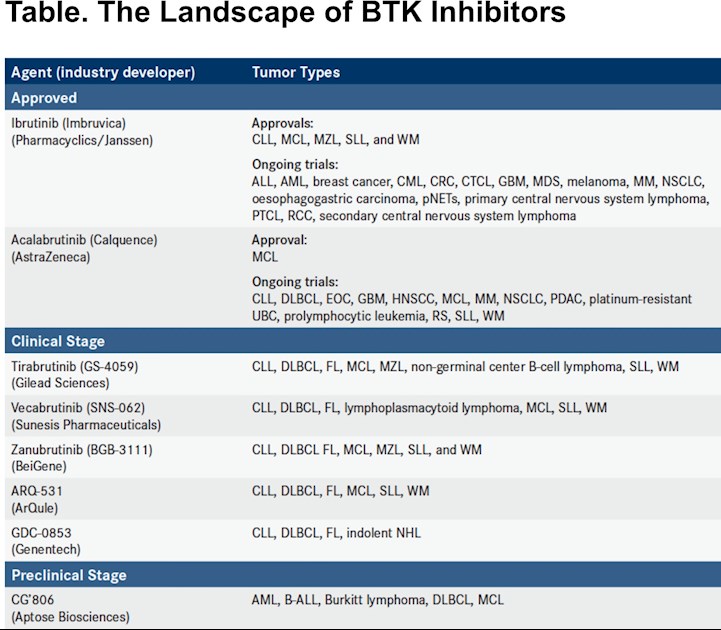
Less than 5 years after Bruton tyrosine kinase (BTK) inhibition was introduced in hematologic malignancies, the need for strategies to address primary and secondary mechanisms of resistance has emerged.
The robust potency of ibrutinib (Imbruvica), which, in December 2013, became the first BTK inhibitor to gain FDA approval, has paved the way for second-generation agents with improved specificity profiles and expanded the potential for adding the drug to novel combination therapies. Ibrutinib use in a wider range of patients has also revealed the significance of resistance mechanisms and the need for options to manage ibrutinib-resistant cancers.
...
Acquired mutations, arising after malignant clone persistence.Recent studies highlight the importance of mutations in BTK and its downstream targets in secondary resistance to ibrutinib.
Two important classes of ibrutinib-resistance mutations have been characterized thus far: the BTK C481S mutation, which abolishes the covalent binding site for ibrutinib, and PLC-gamma-2 mutations.
Whole-exome sequencing of baseline and posttreatment relapse samples from 6 patients with CLL with acquired ibrutinib resistance identified the C481S mutation in BTK, and functional analysis showed that the mutant is reversibly inhibited by ibrutinib.
RNA sequencing analysis in a patient with relapsed/refractory CLL who failed previous lines of therapy and developed ibrutinib resistance identified the BTK C481S mutation as the driver of secondary ibrutinib resistance.
Full article... yes you may have to open an free account...