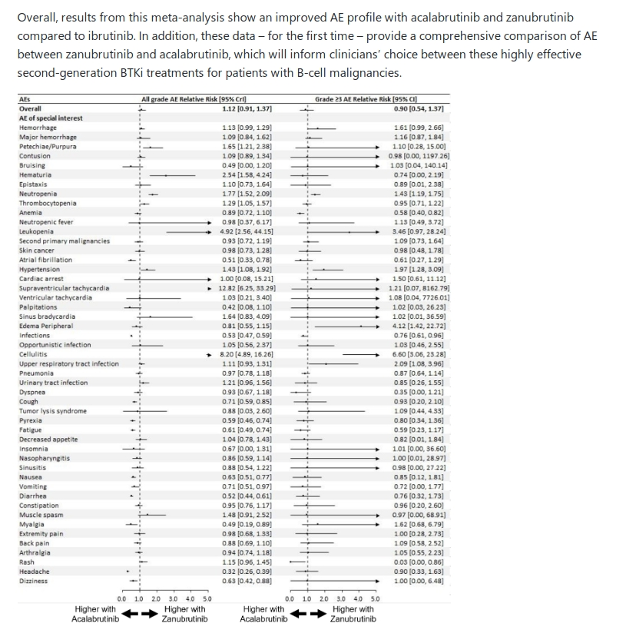
Now that we have a growing choice of BTK inhibitor treatment options, naturally the question is which one is better? Based on this meta-analysis study of the incidence of 84 Adverse Events (AEs) reported in 61 clinical trials involving nearly 7,000 patients, that question should be better framed as "which one best suits my particular health circumstances?". From a 2023 EHA abstract poster presentation, we have the attached table which our specialists can use to determine whether acalabrutinib or zanubrutinib, which along with ibrutinib are currently FDA approved for CLL, is our particular best fit. Ibrutinib might still have the most long term patient data (10+ years for those involved in early trials), plus it only needs to be taken once per day, but zanubrutinib now pretty well matches it for dose adjustment options.
COMPARISON OF TREATMENT-EMERGENT A DVERSE EVENTS OF ACALABRUTINIB AND ZANUBRUTINIB IN CLINICAL TRIALS IN B-CELL MALIGNANCIES: A SYSTEMATIC REVIEW AND META-ANALYSIS
library.ehaweb.org/eha/2023...
As the presentation notes, "Overall, results from this meta-analysis show an improved AE (Adverse Event) profile with acalabrutinib and zanubrutinib compared to ibrutinib. In addition, these data – for the first time – provide a comprehensive comparison of AE between zanubrutinib and acalabrutinib, which will inform clinicians’ choice between these highly effective second-generation BTKi treatments for patients with B-cell malignancies." With CLL being such a heterogeneous disease, this move to personalised medicine can't come fast enough.
Systematic reviews and meta-analyses are considered the highest level of evidence and even though this abstract poster hasn't been peer reviewed, the authors are from Mayo Clinic, NYU Langone Health, Penn State College of Medicine and Peoria - Illinois CancerCare PC, so there's some significant CLL expertise there! In the meta-analysis of PubMed and hematology conference abstracts, "A total of 61 trials were included, involving 6959 patients and 68 treatment arms. Three trials involved randomized comparison between ibrutinib and either acalabrutinib (ELEVATE-RR) or zanubrutinib (ASPEN, ALPINE).
A total of 84 AE were analyzed. Compared with ibrutinib, the average incidence of all grade AE was lower with acalabrutinib (RR=0.74, 95% credible interval [CrI]=0.62-0.85) and zanubrutinib (RR=0.83, 95% CrI=0.71-0.93). In addition, the average incidence of grade ≥3 AE was also lower with acalabrutinib (RR=0.87, 95% CrI=0.63-0.99) and zanubrutinib (RR=0.78, 95% CrI=0.47-1.02) compared to ibrutinib."
With thanks to CLLerinOz for mentioning this EHA poster abstract library.ehaweb.org/eha/2023... in this recent post: healthunlocked.com/cllsuppo...
EugeneL2 has also recently posted about a First-line therapy of CLL review article by Dr. Michael Hallek, who is the founder of the German CLL Study Group and currently an associate editor of Blood, the premier journal of the American Society of Hematology. The German CLL Study Group research is of particular relevance to those who live in countries with universal health care systems, which pretty well means any member not living in the USA.
healthunlocked.com/cllsuppo...
When treatment is indicated, several therapeutic options exist today and need to be selected. A combination of the BCL2 inhibitor venetoclax with obinutuzumab, monotherapy with inhibitors of Bruton tyrosine kinase (BTK) such as ibrutinib, acalabrutinib or zanubrutinib, while chemoimmunotherapy (CIT) is disappearing as a therapeutic option.
The first line therapy selection table from this review, recommends a choice between acalabrutinib, ibrutinib or zanubrutinib for those who have 17p del or mutated TP53 CLL, with their fitness and IGHV mutation status considered irrelevant.
This is an unlocked post: healthunlocked.com/cllsuppo...
Neil