I was wondering what people's opinion was for FCR vs imbruvica first line for moderate risk (unmutated, +Zap70, normal FISH for me) patients. With my genetics, I should expect a average response ~5 years with FCR which is the recommended standard treatment per current guidelines.
I feel like I am a common sense type of person. When I see Dr Furman bring up issues like clonal selection/evolution. The FCR possibly causing mutations. The possibility of other secondary cancers down the road due to patients living longer now. Bone marrow function reduction ~ a year after. There are small considerations like most likely not needing an iv port. Going through a 6 month chemo regimen would be harder on home life as well.
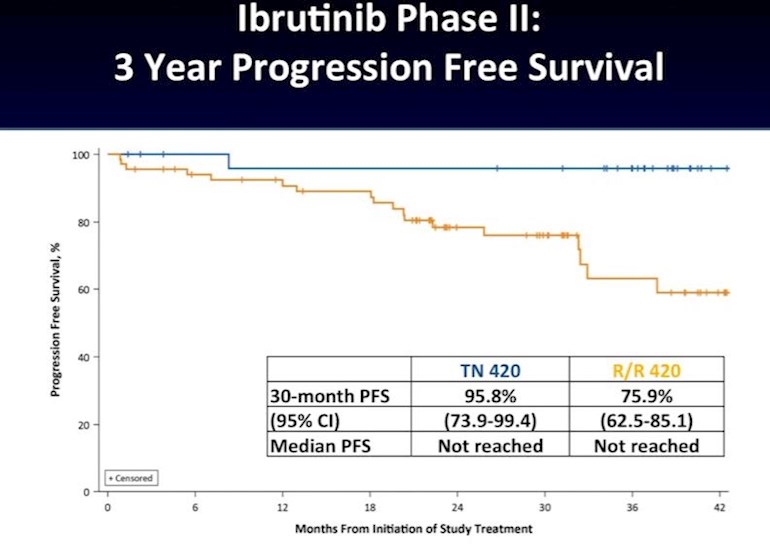
There is a part of me that feels as a a youngerish (45) patient in good health, I would respond well to imbruvica and get many years out of it. I could potentially make it to the completion of a lot of the multi-drug combo trials currently going on. I feel these new combos are going to make a big impact on future treatment guidelines. The picture above is 3 year PFS. The blue line is treatment free, and the yellow is relapsed/refractory patients. 95.8% of first line use is PF after 42+ months.
I am particularly interested in the opinions of patients that have been through FCR and if they would have done it if imbruvica was an option for them. FCR for mutated and 13q is the easier choice. I am meeting with my oncologist soon and this is going to be my main topic of the visit.
youtube.com/watch?v=jNnaBwh... It is a long video, but some great info.
Thanks,
Nathan