Androgen Receptor Signalling Inhibitors – Post-Chemotherapy, Pre-Chemotherapy and now in castration sensitive prostate cancer - Nicholas Mitsiades and Salma Kaochar, Dept. of Medicine and Dept. of Molecular and Cellular Biology, Baylor College of Medicine, Houston,TX 77030. Accepted Manuscript published as ERC-21-0098.R1. Accepted for publication: 15-Jun-2021.
This is an as-yet unpublished manuscript for inclusion in the 2021 publication, Endocrine-Related Cancer, published annually by The Society for Endocrinology.
This particular issue is titled, "Celebrating the 80th anniversary of hormone ablation for prostate cancer". The following excerpt summarizes the main focus of the paper: (Note: ARSI = Androgen Receptor Signaling Inhibitor)
********************************************************
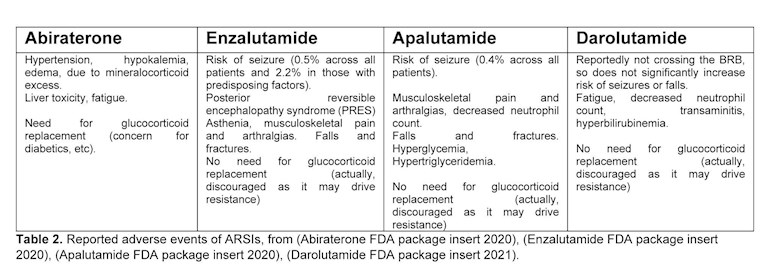
For the purposes of this review, we will focus our discussion on the newer, more effective ARSIs: specifically, the CYP17 inhibitor abiraterone and the 2nd generation AR ligand-binding domain (LBD) antagonists (antiandrogens) enzalutamide, apalutamide and darolutamide, as these are the agents that have entered the market in recent years. Several names have been used to describe these regimens such as ‘Intense Androgen Deprivation’, ‘intensified ADT’, ‘Comprehensive AR axis Targeting’, ‘augmented ADT’, etc. The clinical successes of these combination regimens have led to substantial clinical benefits for PC patients, while validating the old hypotheses in the field about concurrently targeting all sources of androgenic stimulation, and redefining the use of ADT.
******************************************************
For those wanting to understand the current state of SOC treatment options for any state of PCa, this document will be of great utility. It succinctly outlines the evolution of treatments for PCa, from the earlier days of solo-ADT (testicular androgen suppression only) to the more recent drugs that more completely defeat the AR signaling coming from the adrenals and intratumoral androgens. Their review incorporates the clinical evidence for the drugs in current use and the authors' own clinical experience with their patients. They also discuss drug combinations and sequencing as it relates to side effects, mechanisms of resistance (commonly in the form of antagonist-to-agonist switching), treatment strategies to attempt reactivation of the AR transcription, and long-term treatment efficacy. They conclude with a look at what the future might hold for treatment of PCa , esp. in the form of Proteolysis Targeting Chimeras or PROTACs and some other pipeline drugs that might be used in combinations with those already in use.
All good stuff for the SOC "treat-to-failure" community. The 40 page double-spaced paper is in a format understandable by anyone who takes the time to carefully read it. Highly recommended for all patients as a informational guide to help them make decisions about SOC treatment options. That said, the following from the final section titled, "General thoughts/reflections on the state of the field" sums up the near universal resistance to the in-depth testing that would more clearly define the known drivers of PCa on an individual basis and the seeming disregard for the many debilitating side effect on patient QOL. It says as much as anything in the review about what is incomplete in the way SOC is currently practiced for PCa.
**************************
d) Despite the widespread use of AR targeting as first-line choice for treating advanced PC, it is remarkable that the decision to start hormonal therapy and the choice of the specific hormonal regimen has essentially never been driven by a genetic/genomic biomarker. At a time in Precision Oncology where targeted therapies are chosen for each patient based on matching to activating mutations in their targets, the use of hormonal therapies in advanced PC remains remarkably not biomarker-driven. Review of any genomic dataset from treatment-naïve PC reveals little (if any) evidence to nominate AR as a major therapeutic target. In fact, AR overexpression, gene amplification, mutations, expression of splice variants, etc happen in meaningful frequencies only after the hormonal equilibrium of the PC cell has been perturbed by ADT, when derepressed feedback loops and escape mechanisms try to re-equilibrate the cell’s intracellular signaling balance. In the clinic, we utilize ADT as first-line therapy irrespective of the patient’s baseline serum testosterone levels, AR mutation status, or even whether the tumor expresses AR or not. In fact, we do not even test for AR expression in regular clinical practice, although one could point out that the production of PSA by the tumor is evidence of AR activity (but also greatly affected by tumor burden and thus not a quantitatively accurate measure of AR activity). In other words, the clinical algorithm for making decisions regarding when to start hormonal therapy and which agents to use does not incorporate any assessment of the specific degree of AR dependence or any predictive biomarker of responsiveness of each patient’s PC to hormonal therapy. An explanation for this paradox is that ADT does not treat only PC - it treats the entire prostate epithelial lineage as a whole, and we (the physicians) have accepted that normal prostate function will be sacrificed in the process, just as we (the physicians) consider hot flashes, erectile dysfunction, loss of bone density, etc as unavoidable consequences of ADT. But all these adverse effects add significant morbidity for our patients, which is also becoming more prolonged as their life expectancy increases due to more active therapy. More emphasis on survivorship for ADT-treated patients is needed, and we need clinical trials that will try to mitigate these adverse events such as via intermittent use of ADT+/-ARSI or more refined patient selection. This may at first sound contrary to the point we made above in (a) (“if you initiate ADT, offer the best AR axis suppression possible by adding an ARSI”), but it is actually not. Standard ADT is an incomplete therapy that practically guarantees emergence of CRPC, while the patients still have to suffer the adverse events of androgen deprivation. As an alternative approach, more comprehensive AR axis targeting with ADT+ARSI for shorter periods of time may allow for more definitive control of the cancer that then can be followed by careful withdrawal of hormonal therapy in select cases and under close monitoring. This is similar to the concept of ‘intermittent ADT’, which in recent years has been less popular, after Hussain et al (Hussain, et al. 2013) gave us reasons for concern that intermittent ADT may not be adequate therapy. It is possible though that, just like the ARSIs validated several other old concepts in the last decade, they could also resurrect the concept of cycling between periods of intense therapy and de-intensification. Again, the theme is to look back at older paradigms that possibly had value but previously failed in the clinic due to lack of appropriate pharmacological agents, and examine them again in well-designed, biomarker-driven clinical trials that incorporate ARSIs. (emphasis added for post)
*******************************
The full manuscript is available (at the time of this posting) as a PDF download here:
Androgen receptor signalling inhibitors: post-chemotherapy, pre-chemotherapy and now in castration sensitive prostate cancer - Author(s): Nicholas Mitsiades and Salma Kaochar
erc.bioscientifica.com/view...
Stay informed, Be Safe, & Stay Well - Peace K9
++++++++++++++++++++++++++++++++++++
PS: Other manuscripts from this same publication that also might be of interest to our community (and are also currently available for download) are:
Genome-wide crosstalk between steroid receptors in breast and prostate cancers - Author(s): Jorma J. Palvimo and Ville Paakinaho
erc.bioscientifica.com/view...
Using immunotherapy in prostate cancer patients: more than sipuleucel-T - Author(s): Jamie Tae Wook Kwon, Richard J Bryant, and Eileen E Parkes
erc.bioscientifica.com/view...
And the following ones are abstracts only, as the full document is behind the paywall:
New drug delivery strategies targeting GnRH receptor in breast and other cancers - Author(s): Hany Sadek Ayoub Ghaly and Pegah Varamini
erc.bioscientifica.com/view...
Celebrating the 80th anniversary of hormone ablation for prostate cancer -
Author(s): Paramita M Ghosh and Amina Zoubeidi
erc.bioscientifica.com/view...
Management of locally advanced prostate cancer: appraisal of current paradigms and future directions - Author(s): Maria L Sandoval, Ammoren Dohm, and Kosj Yamoah
erc.bioscientifica.com/view...
Targeted inhibition of the WEE1 kinase as novel therapeutic strategy in neuroendocrine neoplasms - Author(s): Lena Weindl, Imke Atreya, Peter Dietrich, Sabine Neubeck, Markus F Neurath, and Marianne E Pavel
erc.bioscientifica.com/view...
Targeting androgen receptor signaling: a historical perspective - Author(s): Alastair H Davies and Amina Zoubeidi
erc.bioscientifica.com/view...
Therapy considerations in neuroendocrine prostate cancer: what next? - Author(s): Himisha Beltran and Francesca Demichelis
erc.bioscientifica.com/view...
The heterogeneity of prostate cancers lacking AR activity will require diverse treatment approaches - Author(s): Mark P Labrecque, Joshi J Alumkal, Ilsa M Coleman, Peter S Nelson, and Colm Morrissey