We've had several posts in the last 24 hours about the U.S. government's plans to negotiate a price for ibrutinib. I mentioned healthunlocked.com/cllsuppo... , that the only remaining advantage ibrutinib/Imbruvica now has over later generation BTKi drugs is that you only needed to take it once per day. I omitted to mention that there is also independently confirmed evidence that ibrutinib can improve T cell function - I haven't seen this property mentioned for any of the other ~14 competing BTKi drugs approved or in clinical trials for the treatment of CLL. healthunlocked.com/cllsuppo... but that and the greater and longer term patient data is about it in terms of a competitive advantage if you exclude any cost advantage.
I also mentioned the lower discontinuation rate for acalabrutinib found in the head to head acalabrutinib vs ibrutinib clinical trial. Much of that is due to not to disease progression, but because those on ibrutinib found the side effects intolerable.
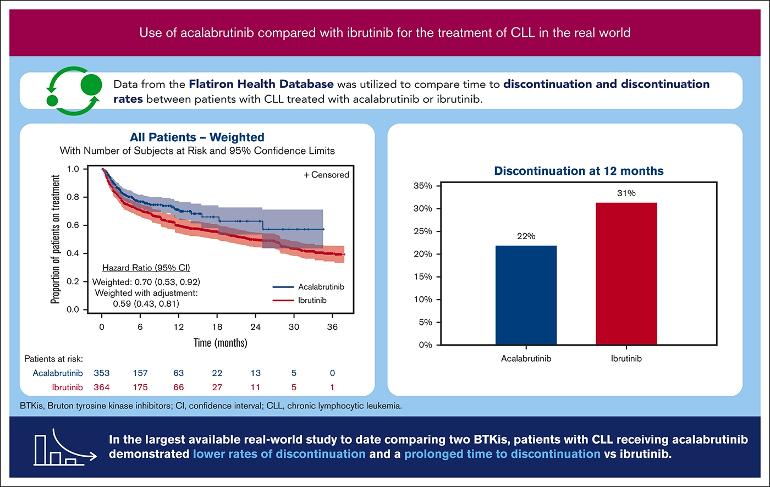
Coincidentally later today, Dr Toby Eyre tweeted about the First comparative effectiveness study of acalabrutinib (Calquence) and ibrutinib (Imbruvica) in real-world patients with chronic lymphocytic leukemia.
The study of 2,509 patients found that "The discontinuation rate at 12 months was 22% for the weighted acalabrutinib cohort vs 31% for the weighted ibrutinib cohort (P = .005). After additional adjustment for prior BTKi use, the acalabrutinib cohort had a 41% lower risk of discontinuation vs ibrutinib (hazard ratio, 0.59; 95% CI, 0.43-0.81; P = .001). In the largest available study comparing BTKis, patients with CLL receiving acalabrutinib demonstrated lower rates of discontinuation and a prolonged time to discontinuation vs those receiving ibrutinib."
ashpublications.org/bloodad...
Neil