Dr Pete Hillmen (UK) reported on this at EHA on 11th June 2021
Background
First-generation BTK inhibitor ibrutinib is a standard of care in CLL/SLL around the world. Zanubrutinib is an irreversible, potent, next-generation BTK inhibitor designed to maximize BTK occupancy and minimize off-target inhibition of TEC- and EGFR-family kinases.
It was hypothesized that the increased specificity of zanubrutinib may minimize toxicities related to ibrutinib off-target inhibition (Coutre Blood Adv. 2019;3:1799-807) and more complete and sustained BTK occupancy may improve efficacy outcomes. Activity and tolerability of zanubrutinib in patients with CLL/SLL has been demonstrated in early phase trials (Tam Blood 2019;134:851-9; Tam Haematologica 2020;epub).
Aims
ALPINE (BGB-3111-305) is a global, randomized, phase 3 study comparing zanubrutinib vs ibrutinib in patients with relapsed/refractory (R/R) CLL/SLL. Here they present the results of a pre-planned interim analysis scheduled approximately 12 mo after the first 415 out of 652 patients were enrolled.
Methods
Patients with R/R CLL/SLL were randomly assigned 1:1 to receive zanubrutinib 160 mg twice daily or ibrutinib 420 mg once daily until disease progression. Randomization was stratified by age (<65 years vs ≥65 years), geographic region, refractory status, and del17p/TP53 mutation status.
The primary end point was overall response rate (ORR) as determined by investigators using the 2008 iwCLL guidelines for CLL and the Lugano criteria for SLL. Sample size was calculated to provide 90% power to demonstrate non-inferiority of zanubrutinib to ibrutinib response ratio at the non-inferiority margin of 0.8558.
A hierarchical testing approach was implemented to test the superiority of zanubrutinib over ibrutinib in ORR if non-inferiority was demonstrated.
Results
Between 5 Nov 2018 and 20 Dec 2019, 415 patients were randomized. Treatment groups were well balanced for demographic and disease characteristics: age ≥65 years 62.3% vs 61.5%, male 68.6% vs 75%, >3 prior lines of therapy 7.2% vs 10.1%, del17p 11.6% vs 12.5%, TP53 mutated without del17p 8.2% vs 5.8%, in zanubrutinib and ibrutinib arms, respectively.
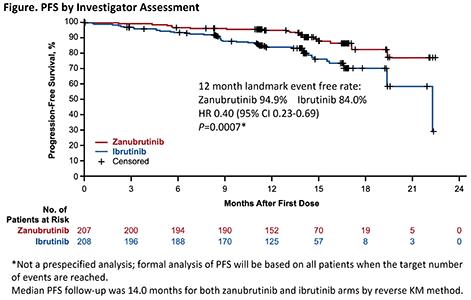
At a median follow-up of 15 mo, ORR was significantly higher with zanubrutinib vs ibrutinib (78.3% vs 62.5%, 2-sided P=0.0006 compared with pre-specified alpha of 0.0099 for interim analysis). ORR was higher in patients with del11q (83.6% vs 69.1%) and del17p (83.3% vs 53.8%) with zanubrutinib, as were overall 12-mo PFS (94.9% vs 84.0%, Figure) and OS rates (97.0% vs 92.7%).
The rate of atrial fibrillation/flutter, a pre-specified safety endpoint, was significantly lower with zanubrutinib vs ibrutinib (2.5% vs 10.1%, 2-sided P=0.0014, compared with pre-specified alpha of 0.0099 for interim analysis).
Rates of major bleeding (2.9% vs 3.9%), and adverse events leading to discontinuation (7.8% vs 13.0%) or death (3.9% vs 5.8%) were also lower with zanubrutinib. Rate of neutropenia was higher with zanubrutinib (28.4% vs 21.7%), while grade ≥3 infections were lower with zanubrutinib (12.7% vs 17.9%).
Conclusion
In this interim analysis of a randomized, phase 3 ALPINE study in patients with R/R CLL/SLL, zanubrutinib was shown to have a superior response rate, an improved PFS and a lower rate of atrial fibrillation/flutter as compared with ibrutinib.
These data confirm that more selective BTK inhibition, with more complete and sustained BTK occupancy results in improved efficacy and safety outcomes. (Trial NCT03734016)