Dated 1st September 2019
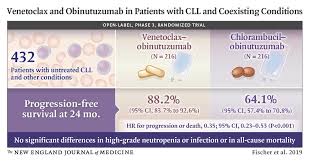
In a phase III trial of patients with previously untreated CLL and co-existing comorbidities, a fixed-duration regimen of obinutuzumab plus venetoclax reduced the risk of disease progression and death by 65%, compared with obinutuzumab plus chlorambucil.
The findings were presented at the 2019 American Society of Clinical Oncology annual meeting by lead author Kirsten Fischer, MD, of the University Hospital of Cologne in Germany.
After a median follow-up of 28.1 months, the investigators found that patients who received obinutuzumab plus venetoclax had significantly higher rates of progression-free survival (PFS) at 24 months, compared with patients who received obinutuzumab plus chlorambucil: 88% vs. 64%. Overall response rates also were higher in the venetoclax group (85% vs. 71%).
The PFS benefit was observed in the venetoclax group, regardless of TP53 and IGHV status, the investigators added. In addition, more patients in the venetoclax group achieved measurable residual disease (MRD) negativity, measured via polymerase chain reaction assay and next-generation sequencing, at three months following treatment: MRD in peripheral blood: 76% vs. 35% (p<0.0001) and MRD in bone marrow: 57% vs. 17% (p<0.0001).
WOW, just WOW for this group of patients!! 

More here: ashclinicalnews.org/on-loca...
Jackie