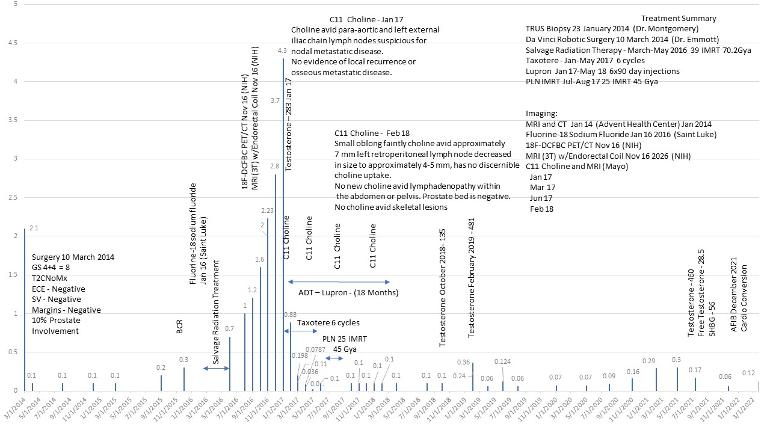
Thought this was interesting. When I saw the Director of Urology at a major NCCN institute in late 2016 for a 2nd opinion after surgery and SRT failed and I argued my case for triplet therapy, he said, no, he would only treat me with monotherapy, ADT, Lupron. Needless to say, I thanked him, left, went to Mayo, the rest is history, and at least in my specific case, has had what I believe is a better outcome than had I done monotherapy.
Enough is No Longer Enough - urotoday.com/center-of-exce...
The treatment landscape for metastatic hormone-sensitive prostate cancer (mHSPC) has become increasingly complex during the last several years. Since 2014 we have been aware that combination treatment with androgen deprivation therapy (ADT) and docetaxel chemotherapy is associated with improved overall survival in patients with mHSPC. Quality of life (QOL) data suggested that better control of the disease with intensified treatment was associated with similar QOL by 12 months after starting chemohormonal treatment. However, there remained a need to ensure that patients who were not candidates for chemotherapy, or who might not benefit sufficiently from chemotherapy, had an option to improve outcomes.
Data providing an option for a broader population of patients followed soon thereafter, with LATITUDE and STAMPEDE both providing evidence that ADT plus abiraterone acetate improved both overall survival and maintained or improved QOL for patients with mHSPC when compared with ADT alone. Subsequent data from ARCHES and ENZAMET demonstrated improved survival with treatment intensification with ADT and enzalutamide, and TITAN demonstrated that ADT plus apalutamide also prolongs survival versus ADT. This left us with four options for use in combination with ADT that were associated with improved disease control and favorable patient reported quality of life outcomes.
More recently, additional data has accumulated from PEACE-1 and ARASENS. These studies demonstrated definitively that in patients eligible for treatment with chemotherapy, the addition of abiraterone acetate or darolutamide to chemotherapy improves overall survival, progression free survival, and additional disease control and toxicity endpoints that are important to patients. All of the patients in PEACE-1, and a majority of patients from ARASENS, had de novo mHSPC. The field continues to debate whether an all-comers, chemotherapy-fit population may benefit from a triplet approach, or whether those who gain the most from including chemotherapy may be patients with de novo metastatic or high volume disease. However, the question of intensified therapy appears to have been definitively answered – combination therapy with ADT plus at least one additional agent is the standard of care.
Ultimately these data fractured the existing ADT-alone dogma that had driven clinical practice for decades, upending the widely held belief that we would do harm to an older and more frail population by doing more. Whether we look at the randomized phase three trials or the patients who have shared their QOL outcomes, we see that this dogma was incorrect. All signs clearly demonstrate that adding something (an androgen receptor signaling inhibitor (ARSI), or docetaxel chemotherapy, or both) to the traditional ADT backbone benefits patients in terms of disease control and quality of life outcomes. With more than a decade of experience using most of these agents, we can hardly say that we are not yet comfortable managing the side effect profiles that they confer. Further, it is disingenuous to suggest that a majority of the patients in our clinical practices have absolute contraindications to the use of an ARSI in combination with ADT. Why, then, have we as a field failed approximately half of our patients and not combined ADT with an ARSI or docetaxel (or a combination of both) in treating their mHSPC? Although I could identify a number of potential reasons, none seem sufficient to justify withholding these treatments from our patients. Rather than scratching our heads, pointing fingers, or debating further, it is time to come together as a group of clinicians, patients, and loved ones and stop the practice of using ADT alone. Our patients deserve combination treatment or a clear understanding of why this is not what will serve them best, and we owe it to them to achieve the outcomes that we know are possible. ADT alone is not enough, and it is time to act on the data and provide the care that every one of our patients deserves.
Written by: Alicia Morgans, MD, MPH, Genitourinary Medical Oncologist, Medical Director of Survivorship Program at Dana-Farber Cancer Institute, Boston, Massachusetts
Published Date: June 2022