Source link :
scr.zacks.com/news/news-det...
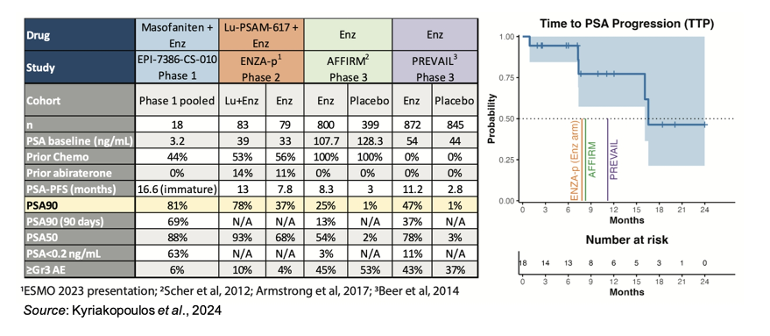
The median PSA baseline for this study was 3.2 ng/mL, which on the surface may look like it is quite different from the other studies listed in the table above. However, this value is for all 18 patients, including the two that were not evaluable for efficacy. For the patients just listed in the waterfall plot above, the median PSA at baseline was 8.5 ng/mL. In addition, if you exclude the post-chemo patients (who had a median PSA at baseline of 2.1 ng/mL), the median PSA at baseline for the pre-chemo patients was 22.8 ng/mL, which is not far from the 33 ng/mL reported in the ENZA-p study. Lastly, the 22.8 ng/mL baseline PSA for pre-chemo patients is also in line with the ACIS study of apalutamide and abiraterone acetate in chemotherapy naïve patients with mCRPC (Saad et al., 2021). Thus, we believe the cross-trial comparisons are appropriate to help put the results seen thus far with masofaniten in context with other therapies in similar patient populations.
PSA response is an important prognosticator, as it was shown to be correlated with a number of positive outcomes from the PREVAIL study (Armstrong et al., 2019). This agrees with multiple other studies of hormone-sensitive prostate cancer (HSPC) patients that show greater PSA responses are associated with better long-term prognoses. In addition, PSA response was shown to be correlated with five-year survival in the PREVAIL study:
• Armstrong et al., 2020: This was a long-term safety and efficacy analysis of the PREVAIL trial that evaluated 5-year survival and its correlation with various pretreatment prognostic factors and post-treatment PSA declines. The results showed that the 5-year survival rate for those with a best overall PSA decline of <0.2 ng/mL was 71% compared to just 11% for those with no PSA decline or < 30% confirmed decline. Even for those who achieved PSA90, the 5-year survival rate was only 42%. This exemplifies the importance of achieving PSA < 0.2 ng/mL and how that can have a positive impact on long-term survival. Approximately 11% (100/872) of patients treated with Enz in PREVAIL achieved PSA <0.2 ng/mL.
PSA responses of <0.2 ng/mL do not appear to be commonly reported in studies of mCRPC patients, however the Phase 3 ACIS study showed that 25% of mCRPC patients treated with apalutamide plus abiraterone acetate and prednisone achieved a PSA level <0.2 ng/mL at any time during treatment compared to 19% treated with just abiraterone acetate and prednisone