(Zoom your browser to read the image)
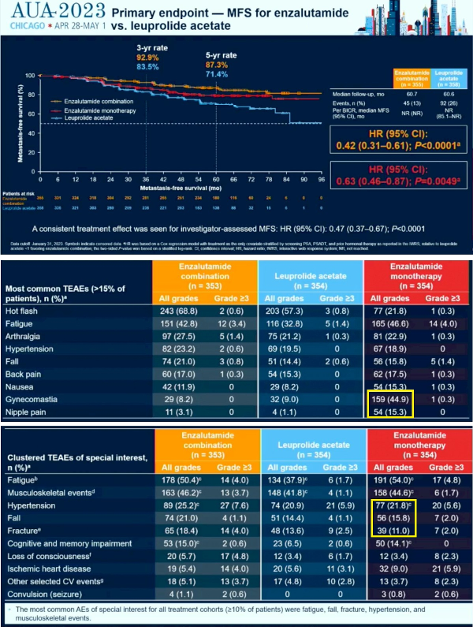
At the AUA2023 the results of the EMBARK trial were presented. This phase 3 randomized trial had three arms and compared in patients with high-risk biochemically recurrent prostate cancer:
- enzalutamide plus leuprolide
- enzalutamide monotherapy
- placebo plus leuprolide
The primary endpoint was metastasis-free survival (MFS). The enzalutamide monotherapy was almost as effective as enzalutamide plus leuprolide with fewer hot flushes, hypertension, falls and fractures. The reduced fractures show that the monotherapy avoids bone loss. Therefore, Xgeva is probably not required. The monotherapy can be used to avoid these side effects.
I added the curve for enzalutamide monotherapy into this image for comparison and included the tables of adverse events. There is increased gynecomastia and nipple pain with the monotherapy. I think this can be avoided by combining it with 20 mg Tamoxifen.