Summary: Dr. Laurence Klotz presented "IHT (Intermittent Hormone Therapy), Quality of Life, and Testosterone Recovery" at the 22nd Annual Scottsdale Prostate Cancer Symposium on Friday, March 17, 2017. Main points include;
a. Intermittent ADT Therapy. No difference in OS found in 10 Phase 3 trials. Men without MET w rising PSA post RT show no significant increase risk over 10 years of OS or TTP while on IAS (intermittent androgen suppression, i.e., IHT).
b. Initial PSA Response Predictive of Outcome. Responders below 0.2 have 75 months median OS vs 13 months for PSA over 4.0.
c. Induction Period (time not treated) study 4 vs 10 months. No difference in OS.
d. Goal for low TET level during initial 1st year of ADT treatment. Patients w fully suppressed TET in 1st year were 2 times (HR =1.90) less likely to develop CRPC (castrate resistant PCa). They also had 10 year Median time to progression!
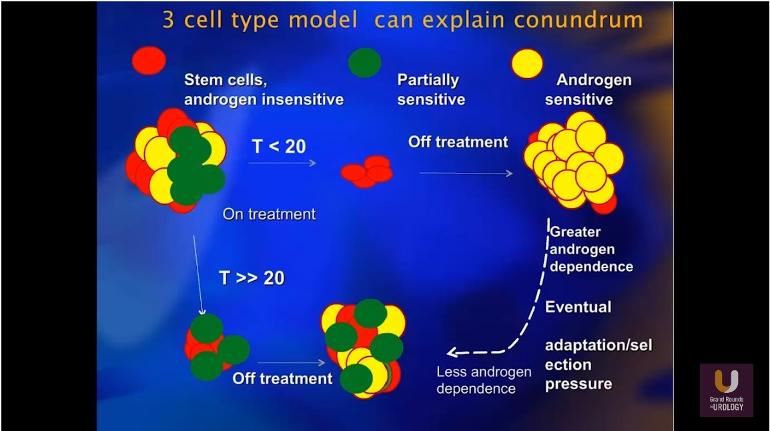
e. 3 Cell Adaptation Model. Theory on how initial ADT treatment w low TET works.
f. Androgen Annihilation. 4 pathway targeting of androgen n TET.
There are some counterindications in this podcast regarding TET therapy and its impacts, especially in the 1st year of ADT treatment. Its important to see the results of the one study posted but the implications from this presentation are clear; higher TET is not protective during the 1st year of ADT…afterwards it appears that intermittent treatment with its introduction of TET, does not appear to affect OS and can improve QOL.
Podcast: MIN Highlights
MIN 1:30 – Developments in past decade (now 16 years ago).
MIN 4:54 – Statins delay progression of men on ADT to CRPC state, 5 years from onset of use; Dr Klotz recommends all men on ADT use Statins. (Probability drops from about 36% to 22%, but ‘Men at Risk’ appears the same for both groups at 11%. Not sure how Statins help here).
MIN 6:31 – Intermittent ADT Therapy. 10 Phase 3 trials for Intermittent Androgen Suppression (IAS); consistent results showing no difference in OS (overall survival) or Time to Progression (TTP).
MIN 7:25 – Study NE Journal Medicine, Sep12, IAS for Rising PSA after RT (radiation therapy); no MET patients. 1500 men, 10 year follow up; no difference OS w HR (hazard ratio) of 1.02 and a “p” value of 0.009!
MIN 8:48 – Study NE Journal Medicine, IAS for Rising PSA in MET patients. 1500 men followed 15 years. HR increases to 1.09. Continuous ADT almost reached significance. IAS (intermittent Androgen suppression) not recommended for men w MET disease.
MIN 10:01 – Meta-analysis IAS studies; 5767 patients. Shows no difference in OS, PFS, CSS n Non PCa Mortality. (But, ”p” values are very poor w only Non PCa Mortality having a P=0.07 n HR 0.90).
MIN 10:30 – PSA Response Predictive of Outcome. PSA at end 7 month induction n OS. PSA responders below 0.2 have 75 months median OS vs 13 months for PSA over 4.0. Note the “p” value of 0.0001!
MIN 11:00 – TET Recovery. Majority (75%) of men recover TET after suspension of ADT within 6 months. This percentage reduces (not defined) with each Cycle of IAS.
MIN 12:18 – Induction Period (time not treated) study 4 vs 10 months. No difference in OS between time of off treatment in the two groups. P=0.38 (38% chance conclusion is wrong). Recommend; treat to PSA nadir below 0.1 then stop.
MIN 14:17 – IAS impact on CV events. Intermittent ADT treatment increase cardio vascular (CV) events over 10 years by 7 % (24-33%, with P=0.02 but HR 0.69). Nor replicated elsewhere.
MIN 16:40 – Goal for low TET level during initial 1st year of ADT treatment. Study of 626 patients. Patients w fully suppressed TET below 20 ng/dL in 1st year were 2 times (HR =1.90) less likely to develop CRPC (castrate resistant PCa) than those with TET higher than 50 ng/dL. P=0.015; 1.5% chance results are wrong. They also had 10 year Median time to progression! Conclusion; initial high TET is not protective over CRPC outcomes.
MIN 18:31 – 3 Cell Adaptation Model. Theory on how initial treatment w low TET avoids creation of heterogenous cancer n delays development of CRPC conditions.
MIN 20:05 – Androgen Annihilation. 4 pathway targeting of androgen generation and TET production. Using Abiraterone, Enzalutamide with Prednisone along with ADT in the STAMPEDE Trial.
MIN 23:02 – Bone Density. Loss bone mass a known side effect of ADT. Aledronate 70 mg/week showed spine, femoral and hip increases in bone mass density (BMD).