In this post I wish to do a very brief discussion on drug trials and drug trial results. In the discussion that follows I have generalised and avoided specifics.
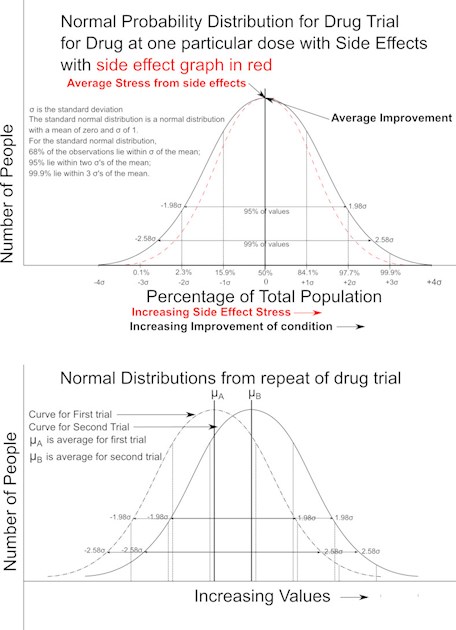
In the image there are two sets of graphs. The top set Graphs A looks at the results from a drug trial and the effects of a drug at a particular dose on a large number of people. There are two curves: one of improvement of condition, and the other of side effect stress. If you look at a packages that drugs come in there is a whole list of side effects. What is not mentioned is side effect stress. Side effect stress is the stress produced on the body’s engineering system by the drug, its breakdown products and the stress from physical and mental health side effects. These list of stresses are often not considered when discussing side effect issues.
The two curves of Graphs A follow a standard normal distribution shape. The two curves when considered together are confusing. From the curves many people can make the mistaken assumption that there is a relationship between side effect stress and improvement of condition. This is not necessarily so. The graphs are the results obtained from the same population of people. However, different people can give a different side effect stress value and improvement of condition value which have no relationship. When the population results are plotted a false relationship can appear to hold true. The graphs do not identify the people the values are taken from. Each person is represented as a point and a best fit curve is drawn through or near the points.
The lower set of Graphs Graphs B shows what happens when the same drug trial is repeated with a different population of people. You get a different set of results. The different set of results may be due to a different underlying condition present or may be due to statistical randomness.
Drug trial results need to be approached with a degree of scepticism. You as the patient need to ask where on the curve of results do I lie?
There is talk of the randomised control trial in medicine. Two articles have a take on this issue
On p66 of the March 2019 edition of Scientific American there is an article titled “Oh, Chute Someone finally did a study on the efficiency of parachutes
scientificamerican.com/arti...
Another article worth reading
npr.org/sections/health-sho...
“...The study's findings were published in the traditionally lighthearted Christmas issue of the medical journal, BMJ.
..."It's a little bit of a parable, to say we have to look at the fine print, we have to understand the context in which research is designed and conducted to really properly interpret the results," Yeh says. Scientists often read just the conclusion of a study and then draw their own conclusions that are far more sweeping than are justified by the actual findings.
This is a real problem in science.
"I know that people often don't look detailed enough into what is being investigated to know how to interpret the results of a trial," says Cecile Janssens, an epidemiology professor at Emory University."
I hope I have given enough information for people to challenge conventional results of drug trials when talking to their doctor.