TAKE ACTION! Tell the CDC that lung cancer patients and survivors need to be placed in Tier 1b to receive a COVID-19 vaccine ASAP. Comments are due by January 26th so act now!
As of January 22, we stand at 24.8 million COVID-19 cases in the US, with 412,936 deaths. The CDC reports that 15.1 million Americans have received at least one dose of vaccine, with 2.4 million people being fully vaccinated.
President Biden and his administration have made addressing the COVID-19 pandemic a top priority and have released their National Strategy for the COVID-19 Response and Pandemic Preparedness. Other steps in the first few days of his presidency include asking Americans to wear masks for the first 100 days of his administration, signing an executive order for a mask mandate on federal property and reenlisting the US in the World Health Organization and joining global efforts to combat the pandemic.
In this week’s update, we will cover several topics including: Call to Action to make your voices heard for the advisory panel making decisions on which groups should be prioritized for vaccination next, new NCCN guidelines regarding vaccination of patients with solid tumors and new vaccines on the horizon.
Call to Action – make your voices heard!
Click here to ask for this: go2foundation.org/advocacy/...
Alongside other lung cancer advocacy groups, we urge you to write to the CDC’s Advisory Committee on Immunization Practices (ACIP) about the need for patients with cancer to be prioritized for vaccination against COVID-19. Most states do not include patients with cancer in Tier 1b.
Please note that comments will be posted publicly on Regulations.gov. Act fast! Comments are due by January 26th before the ACIP meets on January 27th, 2021.
As patients with lung cancer, are there any special considerations for receiving the COVID-19 vaccine?
The National Comprehensive Cancer Network® recently released guidelines for vaccination of patients with solid tumors.
•Patients with cancer should be prioritized for vaccination (CDC priority group 1b/c) and should be immunized when vaccination is available to them.
•Immunization is recommended for all patients receiving active therapy. Patients and their treating physicians should be aware that there are limited safety and efficacy data in these patients.
•Patients who are receiving immunotherapy, targeted therapy, chemotherapy, or radiation therapy should receive the vaccine as soon as it is available. In patients who are undergoing surgery, date of vaccination should be separate from the day of surgery by at least a few days.
New Vaccines on the Horizon
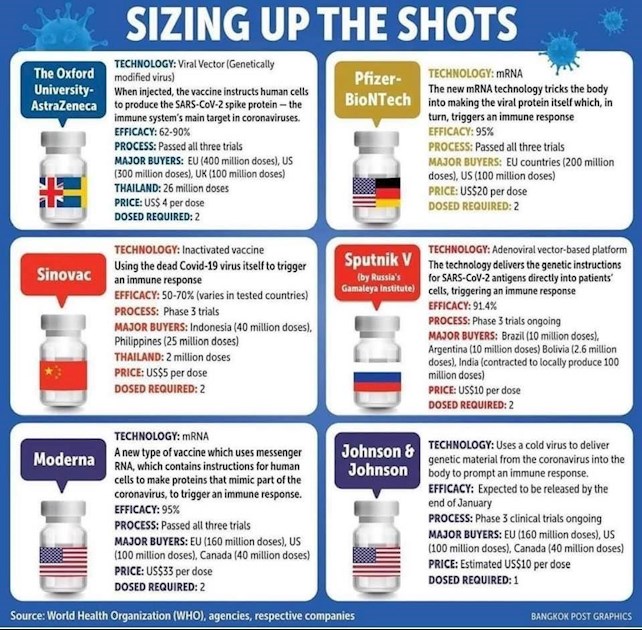
Currently, two vaccines (Pfizer and Moderna) are approved for use in the US. Both these vaccines are mRNA-based. But other vaccines are on the horizon and are already being rolled out in different countries around the world. This tracker from The New York Times offers a comprehensive overview of all vaccines currently in development. This article also summarizes the top three vaccines expected to move forward in the US in the near future. Both the AstraZeneca (AZ) and Johnson & Johnson (J&J) vaccines are built on an adenovirus backbone – this is using a typically harmless virus as a “Trojan horse” to deliver the genetic sequence for the SARS-CoV-2 spike protein to stimulate an immune response. The AZ vaccine will still require two doses while J&J’s will be a single dose vaccine. Novavax’s platform uses a virus like particle (VLP) coated in genetically engineered coronavirus proteins to stimulate an immune response.
This graphic summarizes some key features of current vaccines that are being used globally: