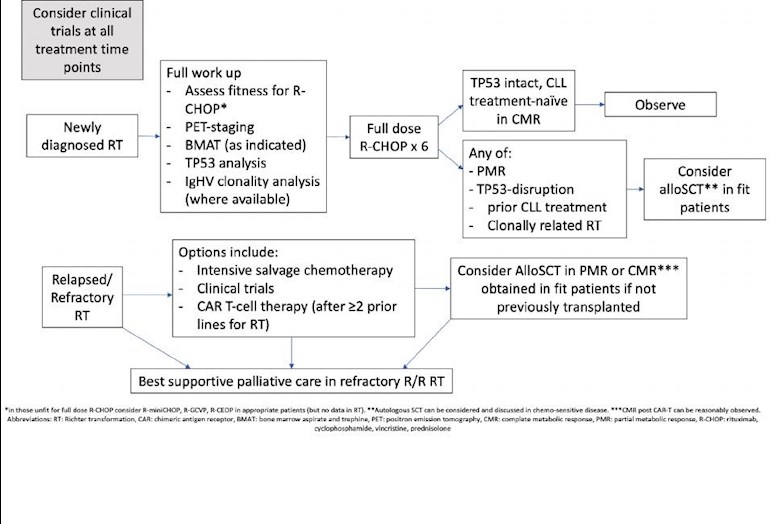
This best practice paper, written by some of the best and most respected CLL experts in the UK and published on 4th October 2021 following a review of all the evidence and clinical trials available, should form the basis for the treatment of Richter's Syndrome in hospitals in the UK.
Details here: onlinelibrary.wiley.com/doi...
Summary:
Each year between 2-15% of CLL undergoes Richter Transformation and 80% of transformations are clonally related to the CLL. Most CLL transforms to Diffuse Large B Cell non Hodgkin Lymphoma (DLBCL) of the ABC type. Very rarely it can transform to Hodgkin Lymphoma or a very aggressive Burkitt's Lymphoma.
Unfortunately, despite targeted therapies being used in trials, the backbone of treatment of Richter's remains R-CHOP although the disease is usually resistant to chemotherapy with a mean overall survival of only 8-10 months. Treatment is difficult because patients are generally elderly, unwell and with several co-morbidities including poor renal function, often with prior chemotherapy exposure. The burden of CLL tumour load also complicating
This paper noted that patients with TP53 mutated (inc 17p del) had worse prognosis. For patients with a clonally unrelated Richter's transformation the survival curves flat line at 48 months, offering some hope for this group of long term survival.
Two prognostic score systems predict overall survival (OS) in the review.
Five factors independently correlated with shorter survival: performance status > 1 LDH >1.5 x upper limit of normal, platelets <100 10/9/l, tumour bulk >5 cm, and more than one prior therapy. When stratified into four groups according to these factors, median OS ranged from 0.33 to 1.12 years.
The second prognostic system used performance status (>1) , achievement of complete remission (CR) with induction therapy and alsoTP53 status. Median OS was 8 months for high risk and 25 months for intermediate risk and the 5 year survival was 70% for low-risk patients (PS =0, CR with induction therapy and NO TP53 disruption). SCT is not recommended for this group but careful monitoring. CAR-T is available in the UK as a therapeutic option for patients who have failed two lines of DLBCL treatment.
There is a UK trial for Richters - STELLAR trial which offers Acalabrutinib as part of the trial. bepartofresearch.nihr.ac.uk...
Jackie