I'll post this. It's tangentially related to our disease. From The Guardian:
Children in England with fatal condition to get world’s most expensive drug.
theguardian.com/society/202...
"Children in England with a rare and fatal genetic condition will soon be able to get the world’s most expensive drug after NHS bosses negotiated a substantial cut in its £2.8m price."
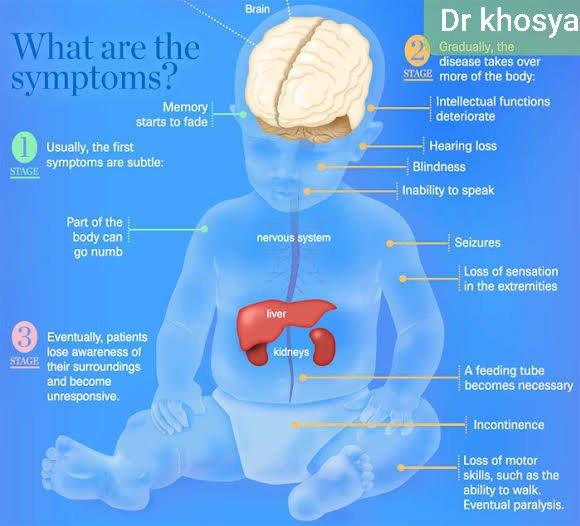
I have read about metachromatic leukodystrophy before, from The Guardian - The symptoms:
"The inherited neurodegenerative disorder severely damages a child’s nervous system and organs. Sufferers experience progressive disability similar to that caused by multiple sclerosis. Symptoms include spasticity, seizures and difficulty talking, swallowing, hearing and seeing."
Reminds me of our lovely malady.
The drug, Libmeldy, from WIkipedia:
en.wikipedia.org/wiki/Atida...
"Atidarsagene autotemcel, sold under the brand name Libmeldy, is a gene therapy treatment for metachromatic leukodystrophy (MLD) developed by Orchard Therapeutics. It contains an autologous CD34⁺ cell enriched population that contains haematopoietic stem and progenitor cells transduced using a lentiviral vector encoding the human arylsulfatase A (ARSA) gene."
Which all makes perfect sense to me, having spent years now reading scientific papers related to AMN/ALD.
And, there is a lentiviral vector therapy for ALD:
creative-biolabs.com/gene-t...
pubmed.ncbi.nlm.nih.gov/223...
This Libmeldy hasn't properly finished trials, and it's effectiveness is questioned.
MLD affects 1:40000 kids, ALD (and I presume AMN), 1:20000.
It is odd that they have approved it now, just as the UK it about to hit one of the biggest phases of austerity since the last round of austerity. Frankly, I'll believe it when I see it. My old mum can barely book a doctor's appointment as it is.
That said, with such few numbers, they wouldn't have to prescribe much.
A victory for any leukodystrophy has to be a victory for us all.
Update:
Libmeldy carries a one-off price tag of £2.8 million, and it claims to offer a permanent cure for MLD. Who could put a price on a child's life? Well, plenty of governments as it goes. But not for this disease.
The BBC has done a nice write-up:
And there a quite a few of these one-dose gene therapy medicines available.
Zolgensma, for spinal muscular atrophy (£1.795 million per single dose) is available on the NHS.
en.wikipedia.org/wiki/Onase...
Zynteglo, for beta thalassemia ($1.8 million price tag), EC approved, turned down by the NHS.
Which brings me to ALD.
Skysona (projected a price tag of up to $700,000 per patient. A bargain). EC approved. UK approval withdrawn.
Skysona was reviewed as part of the European Medicines Agency’s Priority Medicines scheme (PRIME) and was previously granted Orphan Medicinal Product status. The marketing authorization is valid in all 27 member states of the EU, as well as Norway, Liechtenstein, and Iceland.
Good to see the Brexit dividend paying off for the UK.
en.wikipedia.org/wiki/Eliva...
ema.europa.eu/en/medicines/...
nejm.org/doi/full/10.1056/N...
All the above, to be administered the younger the better. Anything that can be administered on demand and obviates the need to find a bone marrow donor is fine by me.
No adult clinical trials of Skysona that I can find. I'd certainly volunteer for one.
I'll gladly welcome additions/corrections to the above. I haven't been following these new gene therapies as closely as I should. We have plenty of other medicines in the pipeline.