out of 68 known Variants that escaped treatment darolutamide is effective against all but one. This article is from june 2021.
it is a invitro study so it needs vitro study, then insilico AI to further confirm but it is still important.
And even shows resistant variants to abiraterone, corticosteroids, estrodiol, all the lutamides.
ncbi.nlm.nih.gov/pmc/articl...
Evaluation of Darolutamide (ODM201) Efficiency on Androgen Receptor Mutants Reported to Date in Prostate Cancer Patients
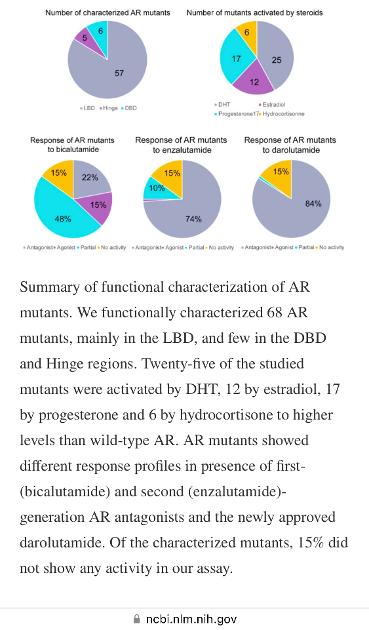
In summary, out of 68 experimentally evaluated AR mutants (24 reported in the previous works and 44 presented in the current study), 25 demonstrated enhanced activation by DHT, 17 by progesterone, 12 by estradiol and 6 by hydrocortisone, compared to the wild-type receptor (Figure 4). The first-generation bicalutamide behaved as a partial or complete agonist for the majority, 43 out of 68, of studied AR mutants (63% of mutants). The second-generation antiandrogen, enzalutamide, demonstrated full or partial activation of eight mutant variants, while the structurally distinct and most recently approved darolutamide demonstrated significant activation in only one mutant at concentrations up to 25 µM [21], which identifies a sequencing opportunity for this drug in men with progressive CRPC with a gain-of-function mutation in the AR under selective pressure of first-line ARPIs.
5. ConclusionsEmergent AR mutations in men with advanced PCa treated with ARPI promote CRPC progression. The incidence of AR mutations was estimated to be around 15% for CRPC patients [4] and the availability of circulating tumour DNA assays now provide a sensitive method to serially detect (and treat) the emergence of resistant AR mutants. This current work expanded the list of experimentally evaluated AR mutants with 44 additional examples (bringing the total to 68) and quantified their response to four major endogenous steroids and three clinically used AR antagonists, including darolutomide. Among these, only darolutamide demonstrated complete inhibition of 67 out of the 68 studied AR mutant variants, with no significant signs of partial of full activation at even higher concentrations.