This is a phase three study results for patients with metastatic castration resistant prostate cancer the vision study . It was published 8 September, 2021 find doctor George Daniel professor of medicine Duke Cancer Institute these are excerpts from the 11 minutes 6 seconds YouTube presentation found at the following link.
youtu.be/Gq2kM4BWD3E?list=P...
My Conclusions: The reviewers finding...
a.Treatment vs Cure: LU177 remains a treatment for castration resistant metastatic prostate cancer . It does not appear to be a cure. This phase three treatment is being provided to advanced castrate resistant metastatic prostate cancer patients. There has been no testing of this procedure on less critically ill , or advanced prostate cancer patients. The best of treatments if implemented too late have different outcomes then if they are introduced earlier. This remains the unknown factor in this new medical modality.
b.OS: For the primary endpoint of overall survival at the end of 23 months there appears to be parity between the two groups, those treated with LU177 and those receiving the standard of care. But at 25 months the standard of care group overall survival dropped to 8% while those treated with LU177 remained and bottomed out at 20% at month 28. It remained at that level of survival until month 32, a 7 month increase. Therefore, a 12% improvement in the reduction of probability for prostate cancer specific mortality, with a 28% increase in time of survival, can be deduced from the randomized collection of data . This is not an insignificant difference.
c.rPFS: For the progression free radiographic survival LU177 is significantly better in the short term. 50% reduction in radiographic progression is still provided at nearly 9 months for LU177 treatment versus 3.4 months for the standard of care alone. This means an additional 6-6 ½ months before half of the participants progressed in producing additional lesions that could be recorded on radiographic testing. But there is a counter intuitive conclusion to this primary endpoint data. The standard of care alone cohort reaches a progression free plateau at 15 months where 12% do not progress . It remains at this level for the remainder of the study. The LU177 group reaches the 12% free from radiographic progression at month 20 ½, 5 ½ months later, but does not stop there. Its improvement then drops and bottoms out at 7% free from radiographic progression. This is an unexpected ending for this treatment and is not explained.
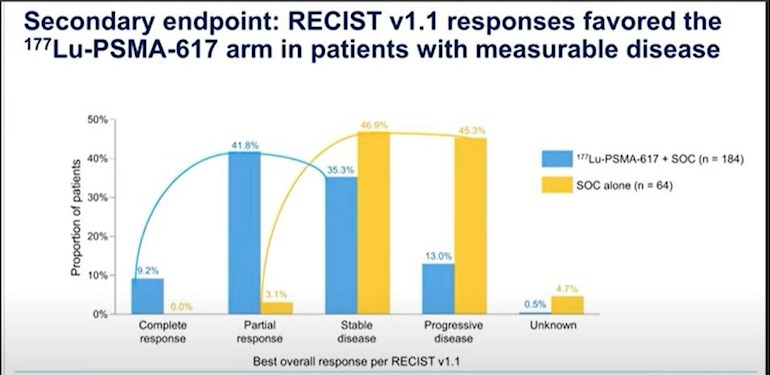
d.Overall Responses: There is no doubt and the graphs for overall survival and radio graphic progression free survival support the fact that responses were much more favorable for the LU177 treatment group than for the standard of care cohort. The shift of responses in the favorable direction, including complete, partial, and stable conditions, heavily favors the LU177 treatment group. Therefore, as limited as the time periods are in which overall survival and radiographic progression free survival are, the quality of life and the overall improvements as measured by response, are much more favorable in the LU177 treatment group.
e.PSA Response: There also is a dramatic difference between the confirmed decrease in PSA count. This decrease would correspond with and have to be produced by the differences in the graphical performance in overall survival and radiographic free survival between the two groups. The reduction in PSA during the limited time periods in which overall survival and radiographic progression free survival are measured, would also mean improved quality of life for participants.
f.Bottom line; if you are a castrate resistant metastatic prostate cancer patient who has undergone every test, hormone treatment and other procedure that is available and have shown no improvement or stabilization of your disease progression, LU177 should be considered. It appears that during the treatment and in the proceeding months that follow, that you will have improved quality of life, reduce your chances of producing new lesions and definitely see a reduction in PSA. It would appear to be worth the effort to determine if at the end of the treatment your case might not be one of the 9.2% of men with a 12% chance for not progressing to prostate specific mortality or radiographic progression.
It remains frustrating to see that this new modality is not being provided or tested on prostate cancer patients with less advanced or critical disease conditions. We can only hope after it's approved for castrate resistant metastatic patients that LU177 can be used earlier in the disease cycle. If it now provides a 12% chance of overall survival and reduction of radiographic progression in advanced cases what will it do if these same patients were to have been treated much earlier in their disease cycle. That question still has to be answered. Meanwhile this is a good last chance best hope treatment when there is nothing left to consider and when all other options have been precluded.