In some recent threads this has come up in context of the PEG shortage and there is some confusion.
For those interested here are some thoughts on MPN interferon versions.
--
There are a many different types of IFNs, with various Greek letters and type classes.
For MPNs only two among these many have historically been used: Type 1, IFN α -2a and Type 1, IFN α -2b.
In the early days these were used as is, under brands IntronA (IFN α -2b) and Roferon (IFN α -2a). These required frequent dosing, even daily in some cases and the early IFN data we have often discussed were partly based on these.
About 25 years ago Pegasys was introduced, and was used mostly for Hep C infections. This new formula was "pegylated" meaning a separate molecule was added to create Type 1, IFN α -2a + Peg. This is where the abbreviation "PEG" comes from. Peg prevents fast clearance of the IFN so it can be dosed less frequently, weekly or even monthly. This allows lower peak IFN levels in the body and thus better tolerance.
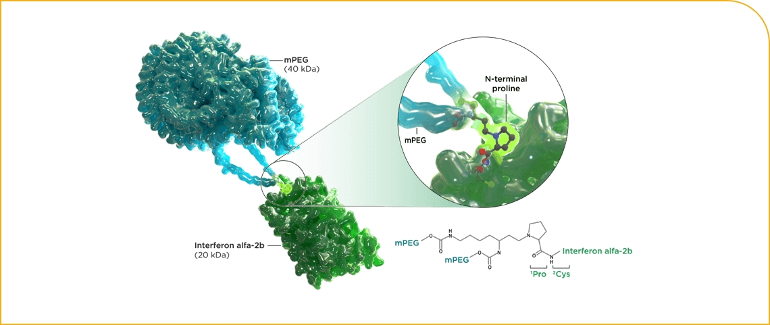
More recently Besremi became available, intended to be an improvement on Pegasys. The pegylation is designed differently and they used a slightly different IFN: Type 1, IFN α -2b + Peg. Note the "2b" in Bes vs 2a in Pegasys. I've posted long ago on this small difference, with unknown implications.
The image here for Besremi shows the old IFN in green, attached to the Peg in blue. So Roferon and Intron A were essentially just the green portion.
One member posting recently has used the early IFN for a long time, and found PEG to be less tolerable. Another member in a thread today had a request denied for Besremi to replace PEG, with Bes asserted to be an "old" medicine. So some Drs should read this post.
--
Intron A is no longer made, but Roferon is. With out-of-the-box thinking maybe Roferon could be a temporary stand in for Pegasys during the production pause. It would require more frequent dosing and care on the dose:
Here is a mention of Roferon, for MPNs, so there should be a reference dosing schedule. It's much lower cost than Bes so maybe easier to get approved for off-label use (ET).
"Interferon alfa (Intron® A, BESREMi® [alfa-2b], and Roferon®-A [alfa-2a]), and sustained-release preparations of these called Peg-Intron and Pegasys® [peginterferon alfa-2a])". (Peg-Intron, like IntronA, is out of production)
lls.org/myeloproliferative-...
This may not be not be practical or reasonable, but could be worth a discussion esp if other options are problematic.
--
As side note, one of the other IFN types, brand name Plegridy, Type 1IFN-β, is sometimes used for MS. It is also pegylated. It was sort of random that IFNα was selected for MPNs, and there has been some suggestion that IFN-β might have provided milder profile. I've posted long ago on this. Their patents covering MS are expired or will soon, maybe they will consider an MPN trial to get a fresh patent but I wouldn't count on it.