Member monarch5000 posted this report in another thread. I figured it's worth a new post with more notes since the title message is worth a fresh look. I posted on an earlier version of the study, link here, but missed this simple conclusion. (See end for context of this plot)
healthunlocked.com/mpnvoice...
--
--
** A key message I get is if you couldn't tolerate IFN before, the combo could do the trick, but if IFN didn't work before it's only somewhat more likely to with the combo.
ncbi.nlm.nih.gov/pmc/articl...
--
Misc notes:
Reduced dosage of both drugs may lead to fewer side-effects compared with monotherapies, hence the top message above.
--
Intolerant get a 2nd chance: "Of 36 patients previously intolerant of PEG-IFNα2, 31 (86%) completed the study" That opens up IFN to a large group to rechallenge with IFN. These pts had good MR (mutation reductions) while pts refractory (it didn't work before) to IFN had less MR. They don't connect being refractory to blood count response, (CHR) but less MR connects to less CHR, see plot and notes on it.
--
Rux dosing criteria: Rux dosage was 5-20 mg BID orally depending on platelet count. Interesting they chose PLT to drive the dose. They do say it was not a dose finding study.
--
MF patients were in an early stage of disease vs RuxoPeg study that had later stage MF.
--
CALR mutation, one had a decrease in allele burden during treatment, two had an increase. So the combo was not so good in this small CALR sample.
--
25% of PV pts discontinued IFN, stayed on Rux, 3% discon Rux. Broadly ~20% quit IFN from adverse events. But conversely it means ~80% stayed on IFN even as a large set were previously intolerant.
--
(22%) patients had mood alterations, including depressive symptoms, agitation, anxiety, and memory impairment. They don't say whether they had to quit IFN nor whether it was reversible, two essential issues.
--
Sjogrens: This one is sort of buried and missing from the AE table, a huge omission. "one patient was diagnosed with Sjögren syndrome. All the drug-related adverse events are known toxicities of either ruxolitinib or PEG-IFNa2". This works out to 2% with Sjo albeit small n limits the meaning. But it seems about what we see in the forum. So the combo did not reduce this dire risk of IFN and thus Sjo is not a tolerance condition to which the title applies. See my post "Last Dose" why this is such a big deal. It further nixes any hope for me and other Sjogies to rechallenege with IFN. No other auto immune (A-I) occurred, another implication of IFN's peculiar association with this normally irreversible and untreatable severe A-I.
--
Big benefit for marrow improvement: (BMHR) in a short time, esp for MF: "19% of PV patients and 33% of MF patients fulfilled the criteria for BMHR" A minority but BM improvement for anyone is great. They say "Long-term treatment with ruxolitinib in patients with MF seems to decrease bone marrow fibrosis in only a small subset of patients" but their citation for this shows a benefit at least equal to the combo therapy, while they caution its reliability either way. See Fig 2 of:
ncbi.nlm.nih.gov/pmc/articl...
Implication of all this is Rux alone or together may be effective for marrow. The combo had BMHR faster than with IFN alone.
--
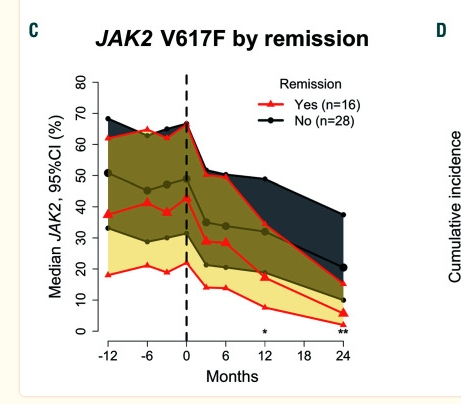
On MR (molecular response) vs CHR: "We found an association between MR and both remission and PBCR" (Complete Blood count response) They use "remission" vaguely to cover various improvements. See plot here, Fig. 3C. The red lines are pts who got PBCR (we know this better as CHR). Much better allele reductions when blood counts respond. They note this matches many prior studies, and has been subject of many posts here. But it's always "association", does one cause the other? (CHR-MR, MR-CHR) Which one? No clear answers.
--
This study was a small n with corresp limitations, and went only two years. But it included PV rather than just MF.