The long awaited successor to FLAIR for patients randomised to one of the Ibrutinib arms is finally ready to open at sites in the UK.
When each site is ready will be up to local researchers and resource availability but the trial will be conducted in 100 NHS Centres in the UK (England, Wales, Scotland and Northern Ireland), the majority of which are already participating in the FLAIR trial.
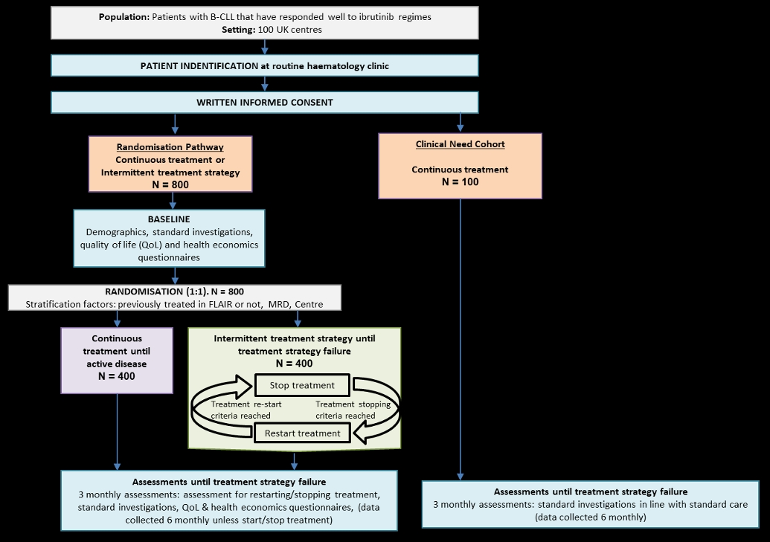
STATIC is designed with multiple pathways, the ‘Randomisation Pathway’ and the ‘Clinical Need Cohort or Group’. The route a participant enters will be determined by their eligibility.
The primary objective is to assess non-inferiority (NI) of the intermittent treatment strategy in terms of time to treatment strategy failure, defined as the first documented instance of active disease (as defined by the 2018 iwCLL criteria) that does not respond to treatment, or death.
Randomisation Trial: A prospective, national, multicentre, open-label, randomised, controlled, 2-arm, parallel-group, non-inferiority, phase III trial to assess whether patients with CLL on long-term treatment with a BTK inhibitor, (including ibrutinib) have similar disease control with an intermittent treatment strategy (experimental arm) compared with standard continuous treatment (control arm).
Clinical Need Cohort: A prospective, national, multicentre, open-label, single-arm, non-randomised cohort to assess the overall safety and survival of patients with CLL receiving long term, continuous treatment with Ibrutinib
The trial will be conducted in 100 NHS Centres in the UK (England, Wales, Scotland and Northern Ireland), the majority of which are already participating in the FLAIR trial.
As of 24th November one site is open to recruitment, Royal Cornwall Hospitals NHS Trust, there are 35 sites in set-up and a further 26 hospitals have been selected and will begin site set-up when they are in a position to do so.
800 participants will be recruited into the Randomisation Trial; 360 participants will be recruited from ibrutinib based arms of the FLAIR trial, and 440 participants will be relapsed refractory patients who have been treated in second or subsequent line of therapy with ibrutinib.
Participants recruited into the Clinical Need Cohort, will have received ibrutinib treatment in the FLAIR trial. It is anticipated that approximately 30 participants will enter this cohort.
In order to reach the required 800 participants in the Randomisation Pathway the trial will recruit for a total of 6 years. Currently no participants have been recruited; potential participants have been approached, and if these participants choose to join STATIC they will consent at their planned routine appointments.