lls.org/sites/default/files...
Just as the virus that causes COVID-19 continues to evolve, so do the recommendations for ways to prevent the infection. As valued partners in the LLS National Patient Registry, we wanted to update you on the latest information and give you our thoughts on staying safe.
Vaccines are the first and best line of defense
The most important step each of us can take is to get all COVID-19 vaccine doses as recommended and encourage those around us to get vaccinated. Pfizer and Moderna (mRNA) vaccines are preferred, but anyone who is unable or unwilling to receive an mRNA vaccine can receive the J&J vaccine.
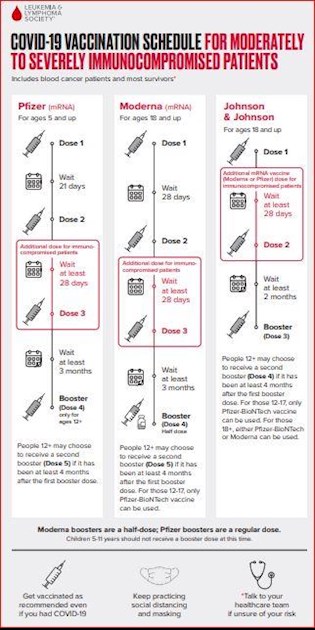
People with moderate to severe immunosuppression (see below for a discussion of who fits into this category) should get four (and may get five) mRNA vaccine doses. The LLS COVID-19 Vaccination Schedule has details on the number and timing of recommended doses for moderately to severely immunocompromised patients:
-
•Three doses as the primary vaccination series, given over the course of about two months
•A fourth (booster) dose at least three months later
•A fifth (2nd booster) dose is also available, and should be given at least four months after the first booster dose
-
Data strongly supports getting the full three-dose series followed by a booster dose. While data about the second booster are still emerging, it seems clear that people with suppressed immune systems will benefit from this additional dose. The vaccines have an excellent safety profile, even with additional dosing. LLS encourages blood cancer patients and survivors to talk to talk to their blood cancer treatment team to decide if the second booster is right for them.
-
A monoclonal antibody called Evusheld can provide another layer of protection
Evusheld can help prevent COVID in people with moderate to severe immunosuppression who may not mount an optimal immune response to vaccination.
Evusheld is an infusion of “ready-made” antibodies that are developed in a laboratory. These antibodies are given by injection and provide at least some immediate protection compared to vaccines, which take a few weeks to work.
However, these ready-made antibodies only last a short time (about six months), while the antibodies that your body makes in response to vaccination tend to last longer. Once your body knows how to make its own antibodies, the levels can be “boosted” with additional vaccines. In addition, vaccines activate other parts of the immune system in addition to antibodies to help protect you.
This is why Evusheld is an extra layer of protection and not a substitute for vaccines.
Evusheld has so far remained active against circulating COVID-19 virus variants including the predominant BA.2 variant, but it appears to be less effective than it was against earlier variants. Just like vaccines, breakthrough infection is possible after receiving Evusheld. FDA will provide guidance in the coming months about the need for repeat Evusheld dosing, which is likely to be recommended if it retains activity against circulating COVID-19 variants.
-
In the meantime, the LLS registry is gathering data that may help inform these decisions. We are tracking registry participants who have notified us that they received Evusheld and who also had previous antibody testing in our study. We will be evaluating their antibody levels five to six months after Evusheld treatment.
-
Who is considered moderately to severely immunocompromised?
Because the immune system is very complex, there is no simple answer to who is moderately to severely immunocompromised. This is why CDC and other policy making bodies provide guidance but recommend that patients consult with their own cancer specialists to determine their level of risk.
-
The world’s oldest and largest private cancer center, Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, has adopted a straightforward recommendation. MSKCC considers all blood cancer patients to be moderately to severely immunocompromised.
-
LLS agrees with the MSKCC approach with one exception. Patients who have had CAR T-therapy that targets the CD-19 protein (Breyanzi, Kymriah, Tecartus or Yescarta) should consider themselves immunosuppressed even if it has been more than two years from their treatment. This is because CAR T is a “living drug,” which may still be active years later, keeping cancer in check but also damaging the body’s immune fighting B cells.
-
LLS strongly encourages all blood cancer patients, regardless of where they are in their treatment, remission or recovery (or “watch and wait” period, which is common for CLL patients) to talk with their blood cancer treatment team about the status of their immune system and whether Evusheld and an additional booster dose are right for them.
-
Can my life go back to normal after I’m fully vaccinated, or I’ve had Evusheld (or both)?
There is no foolproof way to know how well you are protected from vaccines or Evusheld. In people with healthy immune systems, the vaccines remain highly effective in preventing the worst outcomes of COVID, even as new variants circulate. Unfortunately, this may not be the case for some blood cancer patients and antibody tests only tell part of the story.
A high antibody level, while a good sign that your immune system has responded to vaccines, is not the only indication of protection. These tests tell you the amount of antibodies, but not the quality of them. Vaccines also affect more difficult to measure immune responses, including T cell activation, that can protect against COVID.
-
If your cancer healthcare team believes your immune system is compromised because of your blood cancer or its treatment, we urge you to continue taking additional precautions like avoiding large crowds, masking up and maintaining social distancing. This is particularly important as mask mandates are being dropped across the country. Frequent hand washing is always a good idea.
-
Two final notes:
There is no need to get antibody testing after received Evusheld. Because Evusheld is a direct infusion of antibodies, everyone will have detectable levels after treatment.
-
Do not delay getting care if you test positive for COVID-19, even if you have no symptoms, or if you come in close contact with someone who is infected. There are treatments available to reduce COVID severity, but they need to be given as quickly as possible to be most effective.
-
As always, the LLS Information Specialists are on hand to help with up-to-date disease, treatment and support information. Their contact information is here.
-
Sincerely,
Lee Greenberger, PhD
LLS Chief Scientific Officer
Larry Saltzman, MD
Executive Research Director, LLS National Patient Registry