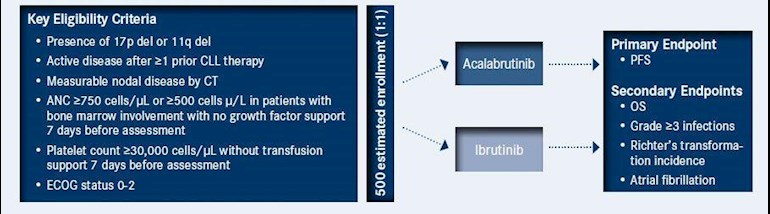
Positive high-level results from the ELEVATE-RR Phase III trial showed AstraZeneca’s Calquence (acalabrutinib) met the primary endpoint demonstrating non-inferior progression-free survival (PFS) for adults with previously treated, high-risk chronic lymphocytic leukaemia (CLL) compared to ibrutinib.
The trial also met a key secondary endpoint for safety, showing patients treated with Calquence had statistically significantly lower incidence of atrial fibrillation compared to patients treated with ibrutinib. Atrial fibrillation is an irregular heart rate that can increase the risk of stroke, heart failure and other heart-related complications.1 Further hierarchical testing revealed no difference for Grade 3 or higher infections or Richter’s transformation. There was a descriptive trend for numerically favourable overall survival. Overall, the safety and tolerability of Calquence were consistent with the profile seen in the broader Calquence clinical development programme.
source: astrazeneca.com/content/ast...
clinical trial: clinicaltrials.gov/ct2/show...