ASCO 2019: Phase 1 Study of Pasotuxizumab, a PSMA-Targeting Bispecific T cell Engager Immunotherapy for Metastatic Castration-Resistant Prostate Cancer
Read the report here
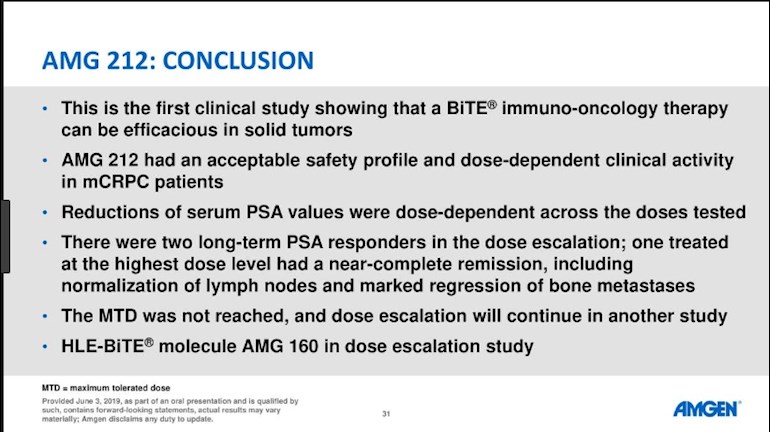
ASCO 2019: Phase 1 Study of Pasotuxizumab, a PSMA-Targeting Bispecific T cell Engager Immunotherapy for Metastatic Castration-Resistant Prostate Cancer
Read the report here
“81% of patients had a grade 3 adverse event”
That’s a horrible safety record
Look on the bright side, there were no grade 5 events. 2 men went home to there family for more than a year in near remission. A number of others were stable. These guys had gone through nearly every available treatment. I think if I had a chance of 25% remission at that stage in the future, sign me up.
NIH definition of a Grade 3 event:
“Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self care activites of daily living.”
They said "... two patients have durable PSA responses beyond one year (14 months and 19 months)." I'm hoping that the word "have" here means that the two patients have gone 14 months and 19 months so far and are still in remission. Maybe it will last a lot longer. This is very encouraging. There was also an implication that, in the next trial, they may go beyond the max 80 unit dose that they used in this trial.
Alan
I agree with p3d1. If it's certain death from cancer vs. 25% chance for 14 months or more, I'd go for the BiTEs (Bi-specific T-cell engagers).
The document didn't say how long the treatment took (one pill or injection? one pill or injection a day for as long as it worked?) or how long the adverse events lasted. Hopefully both treatment and side effects didn't last long.
Alan
Thanks for posting this information....Being PSMA specific, I do wonder if dutasteride--article posted by pjoshea13 today--if given in conjunction with this drug may have gotten even better responses and thus longer remissions--
All the best,
Fish
It appears that any PSMA is sufficient to be targeted. It does not need a large signature. Half of the 16 men where on the low dose. With 2 very good responses thats 25% of the 8 men on the correct dose. We must wait for the phase 2 trial results to get a more accurate picture.
The future must hold a treatment where this type of cell attack and the ARV 110 protac assault will give better results. Hopefully.
Watching the ARV 110--the AR degrader, but there are many trials which look good...We need some drugs that carry less side effects. I watch the macrophage guided therapies and one of those will be in Phase 1 in Europe... I think to slay the beast, it will take a multimodal approach with assist from side drugs like NT-219--Kitov Pharmaceuticals, which can help overcome resistance...or RRx-001--a radio/chemo sensitizer.... the next off label drug to be available may be bempedoic acid--a drug for cholesterol that is an ACLY blocker for tumorigenesis--2019 Europe... Forgot about VERU-111, an alpha/beta tubulin inhibitor... oral and low SE profile so far... combined with radiation ....
Plenty to be hopeful about, brother... The science is coming.... just not fast enough for too many brothers that fall from the Beast's constant attack... Hate this disease !!!!
Don Pescado