I have radically updated this document for many reasons.
First, the proportion who access the document on devices with small screens (phones and tablets) is massive.
Second, technical issues were making it difficult to maintain.
Third, experience of maintaining and using the document has led me to revise my views on many aspects.
Specific changes include the following:
The document page size is now A5 (rather than A4). This makes it much easier to view - for example, on a large-screen phone, turned to view in landscape, the text can easily be read.
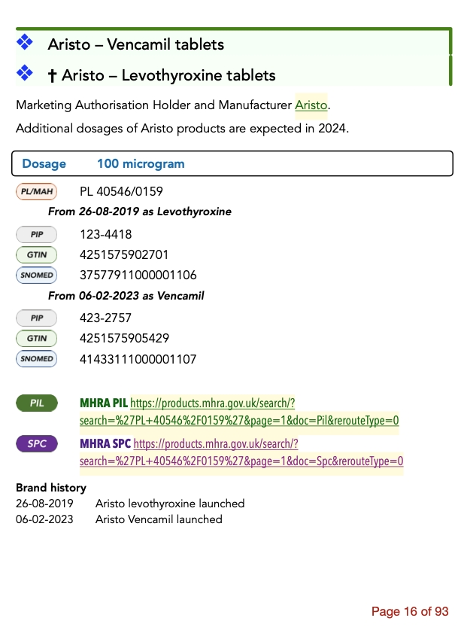
The matrixes which show products, dosages available, and the main excipients discussed, have been reformatted to make the product names more readable.
Details of each product now use graphic signs to indicate what they are.
Details of companies which have previously been repeated for each product (e.g. Advanz appearing three times) have been separated and that duplication has been removed.
Links within the document have been much improved and extended. This should make navigation easier on small screen devices.
Most product details are no longer in tables.
External links have been reduced. In particular:
Links to the electronic medicines compendium (emc) website medicines.org.uk/emc/ change whenever a document is updated and that makes them very hard to maintain. Hence they have been dropped from this release. It has also been noticed that some companies do not supply their documentation to the emc site (e.g. Accord no longer do so, and Teva never have). Further, some documents on emc have not been updated.
Hence the links are now restricted to the companies themselves and the MHRA.
The sections of the document have also been re-ordered. The document starts with the Table of Contents as it always has. But then goes straight into the sections on Levothyroxine tablets, oral solution, injectable and then Liothyronine. All other parts, including the Introduction, are placed nearer the end. Again, this is recognition of its use on smaller devices.
And the details about each product have been moved around bit.
I had been going to wait until the revised document was “finished” before making it live. However, I have decided it makes more sense to move forward now. I need to avoid the problems of maintaining two versions of the document.
At some point, I’ll also be revising my Rest of the World document.
helvella's medicines documents (UK and Rest of the World) can be found here:
helvella - Thyroid Hormone Medicines
helvella has created, and tries to maintain, documents containing details of all thyroid hormone medicines in the UK and, in less detail, many others around the world.
This link takes you to a page which has direct links to the documents from Dropbox and Google Drive, and QR codes to make it easy to access from phones.
The UK document contains up-to-date versions of the Summary Matrix for tablets, oral solutions and liothyronine available in the UK.