Ian Pirker
Ieva Saulite
Johannes von Kempis
First published: 03 January 2021 doi.org/10.1002/acr2.11209
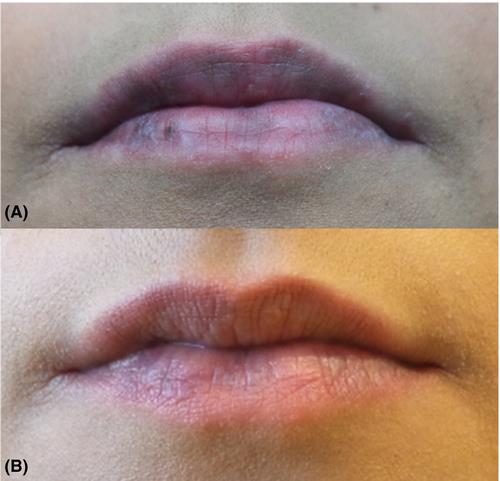
A 23‐year‐old female patient presented with a blue/grey discoloration of the upper and lower lips (A). She had been diagnosed with systemic lupus erythematosus (SLE) 1 year before and by that time, fulfilled the 1987 SLE criteria. Eight months after initiating hydroxychloroquine (HCQ), the patient developed asymptomatic blue/brown macular hyperpigmentation of the upper and lower lips (A). Histological examination revealed pigment incontinence without signs of cutaneous neoplasia or inflammation. A diagnosis of HCQ‐associated hyperpigmentation was made, and topical treatment with tacrolimus 0.03% ointment bds was initiated. During 3 months of topical treatment under continuation of HCQ treatment, hyperpigmentation improved gradually (B). The minimal incidence of HCQ‐associated cutaneous hyperpigmentation has been estimated at approximately 7% (1). Sites of hyperpigmentation involve the legs in most cases but may present at the palate, gums, arms, and face (1, 2). A cross‐sectional study showed an association between HCQ‐linked hyperpigmentation and therapy with antiplatelet agents and/or oral anticoagulants (2). It is unclear whether the incidence of cutaneous hyperpigmentation correlates with the cumulative dose and/or duration of HCQ use (1). In another HCQ‐associated effect, retinopathy is linked to an increased incidence (by 1%) in patients with more than 1000 mg of HCQ/day or in patients with HCQ treatment duration of more than 5 years (3). A French case–control study revealed significantly higher HCQ blood levels in patients with HCQ‐associated hyperpigmentation than in the control group (n = 24), with one patient developing retinopathy (1). Data on the therapy of HCQ‐associated mucocutaneous hyperpigmentation are not available. Improvement of HCQ‐associated skin hyperpigmentation under continuation of HCQ has been reported in 27.3% of patients (1). Even though the discoloration is harmless, discontinuation of HCQ may be considered if cosmetically disturbing mucocutaneous pigmentation occurs. However, because the therapeutic benefit of HCQ in patients with SLE often outweighs cosmetic aspects, topical treatment with tacrolimus may be considered in some patients.
References
1Jallouli M, Francès C, Piette JC, Huong du LT, Moguelet P, Factor C, et al. Hydroxychloroquine‐induced pigmentation in patients with systemic lupus erythematosus: a case‐control study. JAMA Dermatol 2013; 149: 935– 40.
Crossref PubMed Web of Science®Google Scholar
2Bahloul E, Jallouli M, Garbaa S, Marzouk S, Masmoudi A, Turki H, et al. Hydroxychloroquine‐induced hyperpigmentation in systemic diseases: prevalence, clinical features and risk factors: a cross‐sectional study of 41 cases. Lupus 2017; 26: 1304– 8.
Crossref CAS PubMed Web of Science®Google Scholar
3Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF, American Academy of Ophthalmology. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology 2011; 118: 415– 22.
Crossref PubMed Web of Science®Google Scholar
Volume3, Issue1
January 2021
Pages 41-41
References
© 2021 The Authors. ACR Open Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
This is an open access article under the terms of the Creative Commons Attribution‐NonCommercial License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.
Publication History
Issue Online:
17 January 2021
Version of Record online:
03 January 2021
Manuscript accepted:
04 November 2020
Manuscript received:
29 October 2020
SOURCE: onlinelibrary.wiley.com/doi...
LUpus Patients Understanding & Support (LUPUS): lupus-support.org/topic/327...