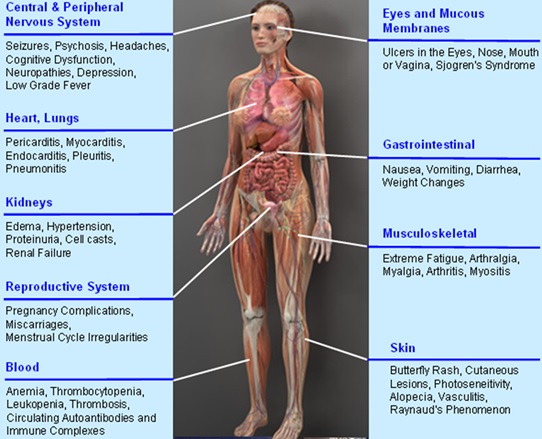
Lupus, a chronic debilitating inflammatory autoimmune disease, impacts 1.5 million people, mostly women, in the U.S. and 5 million worldwide. With a 10 year survival rate of 90%, patients suffer with a disease for which there is no effective treatment. Current drugs like immunosuppressants only provide palliative care by making symptoms easier to live with. These immunosuppressants often come with harmful side effects. Lupus impacts the entire body including skin, musculoskeletal, digestive, nervous and reproductive systems, as well as blood, lungs and heart.
Only one drug has been approved to treat lupus in the past 50 years. Benlysta received the FDA’s blessing in March 2011 and was subsequently acquired and is marketed by GlaxoSmithKline. Sales have been slower than expected due to limited efficacy, among other things. As other drugs for lupus gain FDA approval, the market for their sales is projected to reach $4 billion annually by 2022. Despite strong interest and efforts by big pharma to develop lupus drugs, an effective and safe therapy for this indication remains elusive.
Israel-based XTL Biopharmaceuticals is set to commence a Phase II study of its drug hCDR1 in the treatment of systemic lupus erythematosus (SLE), which accounts for 70% of lupus cases worldwide. If data show efficacy, it would position XTL and its drug very favorably in a market that is in dire need of a safe and effective treatment.
josh_sThe Bio Connection recently spoke with XTL’s CEO Josh Levine about the company’s lupus drug hCDR1.
For more information: