Following on from the interesting discussion on testing for 17p del and TP53 disruption/mutations I thought it might be worth posting this paper which sets out how this testing and analysis is done. It's published by ERIC, the European Research Initiative on CLL, and is the 2024 update of the last recommendations for TP53 analysis in CLL from 2018.
TP53 and 17p del testing is still important in the days of targeted therapies, although less crucial. Unfortunately, decisions regarding the cut off points or thresholds for reporting are not clear cut and based on the current knowledge of the relevance of low-burden TP53-mutated clones, a specific variant allele frequency (VAF) cut-off for reporting TP53 mutations is no longer recommended, but instead, the need for thorough method validation by the reporting laboratory is emphasized.
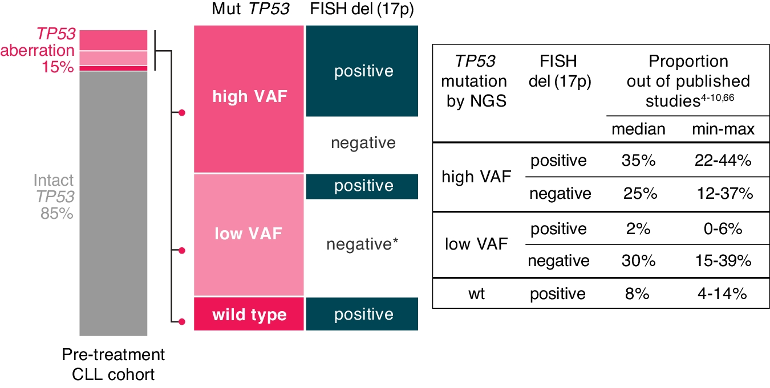
A TP53 aberration is defined as either the deletion of the TP53 gene locus on 17p13 [del(17p)] or the presence of a mutation, i.e., somatic change in the sequence of the TP53gene (TP53mut). The frequency of TP53 aberrations in patients with chronic lymphocytic leukemia (CLL) is higher in those with unmutated immunoglobulin heavy variable (IGHV) genes. Generally, the frequency is low at diagnosis (5-10% of patients, depending on the method used), it is slightly higher in cohorts of patients entering frontline treatment (10–20%; Fig. 1), and further increases in later disease stages, predominantly in chemoimmunotherapy (CIT)-treated patients and Richter transformation (up to 50%) In patients with CLL, del(17p) is mostly accompanied by TP53 mutations, and sole del(17p) is infrequent, while sole TP53 mutations are more commonly found.
The prognostic value of TP53 aberrations is evident early in the course of CLL. Several prognostic scores developed to predict time-to-first-treatment (TTFT) include TP53aberrations as a variable. The CLL international prognostic index (CLL-IPI) incorporate TP53 aberrations as an independent predictor of shorter TTFT. A recent ERIC study and a single centre study from MD Anderson revealed that TP53 aberrations predict TTFT only in patients with unmutated IGHV genes.
TP53 aberrations also have paramount prognostic value in treated patients with CLL since, generally, they confer a worse prognosis with all available treatments, including agents targeting B cell receptor (BcR) signaling and BCL2, at least in the relapsed/refractory setting.
Predictive value of TP53 alterations
The predictive value of TP53 aberrations is clear when CIT regimens are included among the treatment options: in fact, targeted agents as either monotherapy or in combination outperformed CIT regimens in the frontline and R/R settings and represent the preferred option for these patients. Use of CIT allows the TP53 clone to grow and become dominant.
The clinical relevance of low-burden TP53 mutations is still debated. The vast majority of evidence was obtained in the era of CIT, and no clinical trial was designed to assess their impact.
It is recommended assessing del(17p) first and then TP53 testing only in cases without del(17p). However, following this two-step procedure can be difficult and may cause treatment delays, therefore, it is preferred to analyse both TP53 gene mutations and locus deletions simultaneously, if possible.
For TP53 variant detection, the preferred methodology is NGS, but Sanger sequencing can still be used if NGS is not available
The full technical methodology is in the paper, together with more detail regarding the significance of the burden of TP53 mutations.
nature.com/articles/s41375-...
Jackie