A few members who have been recommended ventoclax/Venclexta treatment, have posted about their concerns of developing Tumor Lysis Syndrome (TLS). So hopefully the following report from Memorial Sloan Kettering (MSK), summarising 5 years of experience (2016 to 2020 inclusive), of "616 venetoclax escalations among 136 pts with CLL", will be reassuring. The abstract reports that "none developed clinical TLS."
As the venetoclax safety information states: venclexta.com/important-saf...
TLS is caused by the fast breakdown of cancer cells. TLS can cause kidney failure, the need for dialysis treatment, and may lead to death. Your healthcare provider will do tests to check your risk of getting TLS before you start taking VENCLEXTA. You will receive other medicines before starting and during treatment with VENCLEXTA to help reduce your risk of TLS.
You may also need to receive intravenous (IV) fluids into your vein. Your healthcare provider will do blood tests to check for TLS when you first start treatment and during treatment with VENCLEXTA.
It is important to keep your appointments for blood tests. Tell your healthcare provider right away if you have any symptoms of TLS during treatment with VENCLEXTA, including fever, chills, nausea, vomiting, confusion, shortness of breath, seizures, irregular heartbeat, dark or cloudy urine, unusual tiredness, or muscle or joint pain.
The Venclexta physician information document, rxabbvie.com/pdf/venclexta.pdf states;
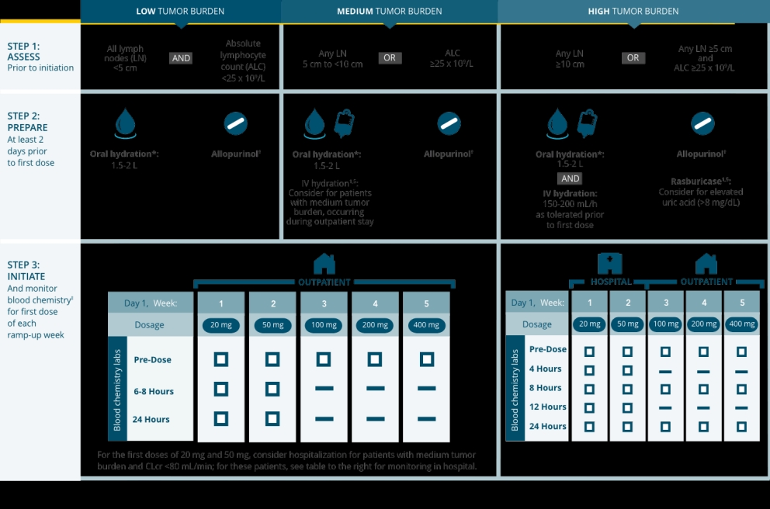
"The risk of TLS is a continuum based on multiple factors, particularly reduced renal function, tumor burden, and type of malignancy. Splenomegaly may also increase the risk of TLS in patients with CLL/SLL.
Assess all patients for risk and provide appropriate prophylaxis for TLS, including hydration and anti-hyperuricemics. Monitor blood chemistries and manage abnormalities promptly. Employ more intensive measures (intravenous hydration, frequent monitoring, hospitalization) as overall risk increases. Interrupt dosing if needed; when restarting VENCLEXTA, follow dose modification guidance."
Section 2.4 of that document includes a section Risk Assessment and Prophylaxis for Tumor Lysis Syndrome.
Now to the abstract from the MSK report of 5 years of experience with venetoclax ramp-up for CLL patients, which also provides one center's experience of the percentage of patients requiring hospitalisation for proactive TLS monitoring, with my emphasis.
pubmed.ncbi.nlm.nih.gov/391...
Venetoclax is a BCL2 inhibitor used in chronic lymphocytic leukemia (CLL) which can cause tumor lysis syndrome (TLS). We aimed to determine the incidence of and risk factors for TLS among patients with CLL/small lymphocytic lymphoma (SLL) who received treatment with venetoclax at our institution from 1/1/2016 to 12/31/2020. We included 616 venetoclax escalations among 136 pts with CLL. 74 pts (54%) underwent escalation exclusively outpatient, 35 (26%) had at least one planned hospitalization and 27 (20%) were escalated exclusively inpatient. During venetoclax initiation, 86% of pts received allopurinol, 71% intravenous hydration, 18% phosphate binders, and 10% prophylactic rasburicase. Among the entire cohort, 7 pts (5.1%) developed laboratory TLS by modified Cairo Bishop criteria and none developed clinical TLS. Incidence of laboratory TLS was 15% for those escalated exclusively inpatient, 2.9% for those with any prophylactic hospitalization and 2.7% for those escalated exclusively outpatient. Those who developed TLS were more likely to have higher TLS risk, and no additional risk factors were identified. In this single institution retrospective cohort study, laboratory TLS was observed, though clinical TLS was not. Prophylactic measures, including use of IV hydration, may have contributed to low rates of observed TLS in the outpatient setting.
The attached graphic illustrates how the risk of TLS is determined and the recommended mitigation steps.
Neil
 My doctor at Stanford at the time (Dr. Coutre RIP) assured me that since I didn't have bulky lymph nodes I likely wouldn't have much in terms of TLS and he was right.
My doctor at Stanford at the time (Dr. Coutre RIP) assured me that since I didn't have bulky lymph nodes I likely wouldn't have much in terms of TLS and he was right.