Trial registration number ISRCTN14197181
bmjopen.bmj.com/content/13/...
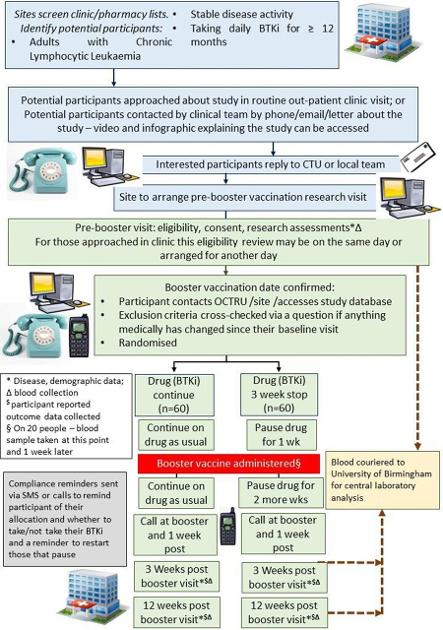
This study aims to determine in patients with chronic lymphocytic leukaemia (CLL) if a 3-week pause in Bruton tyrosine kinase inhibitor therapy (BTKi) starting 1 week before delivery of SARS-CoV-2 vaccine booster, improves vaccine immune response when compared with continuation of BTKi.
Methods and analysis An open-label, randomised controlled superiority trial will be conducted in haematology clinics in approximately 10 UK National Health Service (NHS) hospitals. The sample size is 120, randomised 1:1 to intervention and usual care arms. The primary outcome is anti-spike-receptor binding domain (RBD) antibody level at 3 weeks post-SARS-CoV-2 booster vaccination. Secondary outcomes are RBD antibody levels at 12 weeks postbooster vaccination, participant global assessments of disease activity, blood films, full blood count and lactate dehydrogenase levels, impact on quality of life, self-reported adherence with request to temporarily pause or continue BTKi, T cell response against spike protein and relative neutralising antibody titre against SARS-CoV-2 viral variants. Additionally, there will be an investigation of any effects in those given influenza vaccination contemporaneously versus COVID-19 alone.
I'd sign up for this trial if I could!