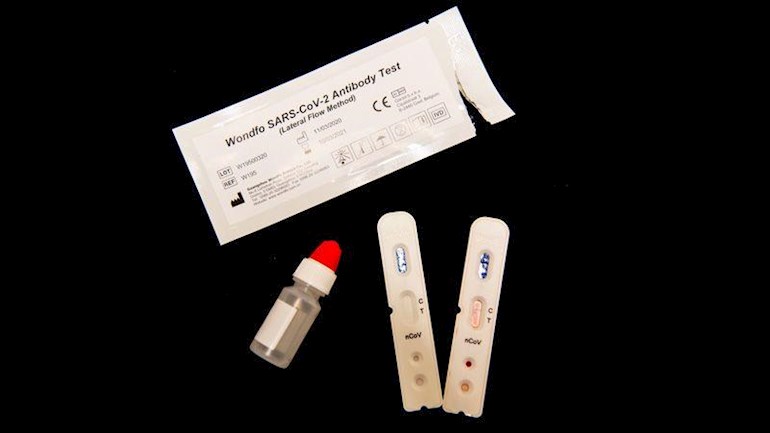
The United Kingdom could begin large-scale testing for coronavirus antibodies within days, government officials have said. If the roll-out goes ahead as planned, the country could become the first to implement at-home testing on this scale — but researchers caution that properly validating the accuracy of such tests and manufacturing them in large quantities presents a significant challenge.
Covert coronavirus infections could be seeding new outbreaks
On 25 March, a UK government official said that the country had ordered 3.5 million ‘finger-prick’ tests and planned to order millions more. The test will analyse drops of blood for antibodies that show whether a person has previously been infected with the coronavirus that causes COVID-19. This will show who might now be immune and aid researchers in understanding the virus’s spread. These ‘serological tests’ should become available to the public in days rather than weeks or months, said Sharon Peacock, director of the national infection service at Public Health England (PHE), a UK health agency. Peacock suggested that the bulk of the UK tests, which will be available to buy from Amazon and pharmacies to perform at home, had not yet arrived.
The blood test will need to distinguish between antibodies against the COVID-19 virus and those against other seasonal coronaviruses to which people are commonly exposed, he says. “I would expect the false-positive rate to be very high because of this prior exposure — unless they figured out how to make the serological test very specific,” he says.
But supply is likely to remain limited, says David Wraith, an immunologist at the University of Birmingham, UK. It will be challenging for companies to manufacture millions of tests and for any one government to secure so many during a global pandemic, meaning that health-care workers must be given priority access, he says.
It is not clear who is developing the UK test. A PHE spokesperson said the agency was talking to a range of companies. Peacock added that highly vulnerable members of the public who test positive will also require further tests before they can resume normal life.
More here: nature.com/articles/d41586-...
Stay safe, Jackie