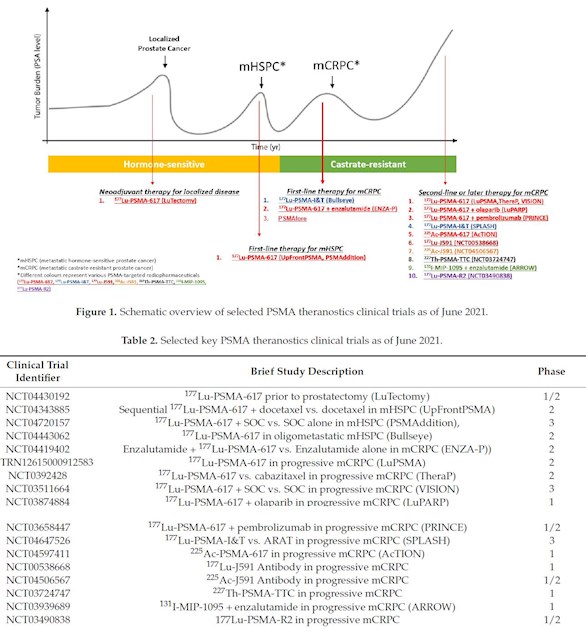
Some forum members are looking for a 177Lu PSMA therapy. Here is an image of the available trials most of them are currently recruiting.
Novartis is offering the PSMAddition trial which is mainly for newly diagnosed high-risk patients with bone metastases. annalsofoncology.org/action... , clinicaltrials.gov/ct2/show... and the PSMAfore trial for metastatic, castration resistant patients who had Zytiga or Xtandi but no chemo yet. clinicaltrials.gov/ct2/show...
This image is from a recent article by Zhang et al. mdpi.com/2072-6694/13/16/40... Here is a link to the image in a bigger size: up.picr.de/42574912ge.png