I am very sorry to have to share the news that NICE, the National Institute for Health and Clinical Excellence, has rejected approval of tocilizumab for GCA. Their decision is open to consultation, and we would like as many GCA patients as possible to comment on their website about this decision.
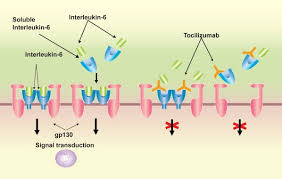
Tocilizumab (TCZ), or Actemra, which has been approved for GCA in the US and Canada, is a relatively new 'biologic' drug, which directly targets IL-6, one of the key 'interleukins' that is a major player in the development of giant cells and the inflammation of the artery linings. The submission for approval was based on the international GiACTA study, which combined weekly infusions of TCZ with a tapering steroid programme. TCZ has been demonstrated to aid tapering in rheumatoid arthritis and reduce the overall 'burden' of cumulative steroid doses. It demonstrated a similar effect for GCA and appeared to cut down rates of relapse considerably.
Here at PMRGCAuk we are disappointed of course, not least because this is the first significant step forward in treating GCA for over half a century! It simply isn't acceptable that patients with GCA should have to languish on yoyoing doses of steroids for years and years, while people with other diseases have a range of alternative drugs to choose from.
NICE have done a (very complicated) calculation and come to the conclusion that TCZ is too expensive, because it won't deliver on 'Quality of Life Years'. One of the parameters of this calculation is their assumption that 'most' patients are elderly. In fact, they have extrapolated from the 'mean' age of onset of 72 the assertion that 'most' people with GCA are over 80! Clearly we are going to challenge this, politely of course, but it would be great if as many people as possible could get involved in the consultation.
To do so, you have to visit the NICE website and register. And read the documents. Then fill in the online consultation form.
nice.org.uk/guidance/indeve...
We only have till 4 January to do this, before the committee meets again to consider the consultation submissions and make their final decision (which btw won't be reviewed for 3 years). Seeing as Christmas and New Year come in the middle we are really pushed for time.
I'll be posting more on this thread but meanwhile please have a look at this link that gives the clearest yet explanation of what TCZ is and what the fuss is all about.
newswire.ca/news-releases/h...
Thanks!
Kate