There are trials of this sort going on, some members are in one. Not sure if this one has been posted. It's from China n = 44 for the full period of 2 years.
"The patients received ropeg at an initiating dose of 250 μg at week 0, followed by 350 μg at week 2, and subsequently 500 μg from week 4 thereafter, if tolerated."
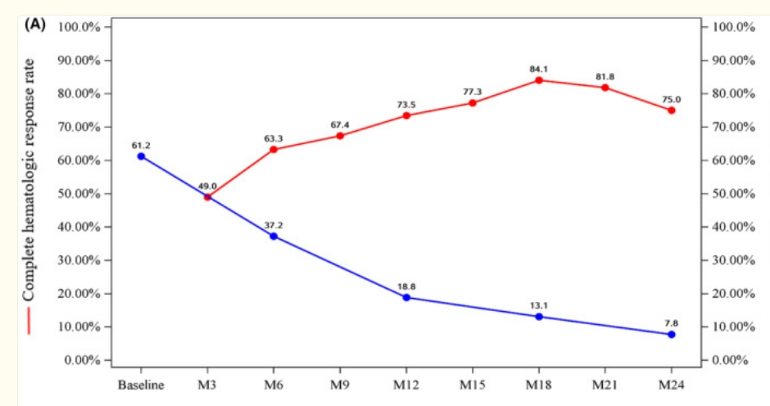
They discuss this plot wherein CHR is being lost but VAF still goes down hence "decoupled" and the title "Molecular remission uncoupled with complete haematological response in polycythaemia vera treatment with ropeginterferon alfa‐2b"
The pts were all HU intolerant. They don't specify whether resistant, which is the higher risk category.
A very good VAF reduction at 2 years, much better than the original Ropeg trial. It's still not clear how these pts widely tolerate such high doses, we see here many members would not, and my tragic outcome likely was from excess dosing. But if the other ongoing high dose trials reproduce this, it is compelling. One missing detail is the non-driver status of the cohort, this could influence the result.