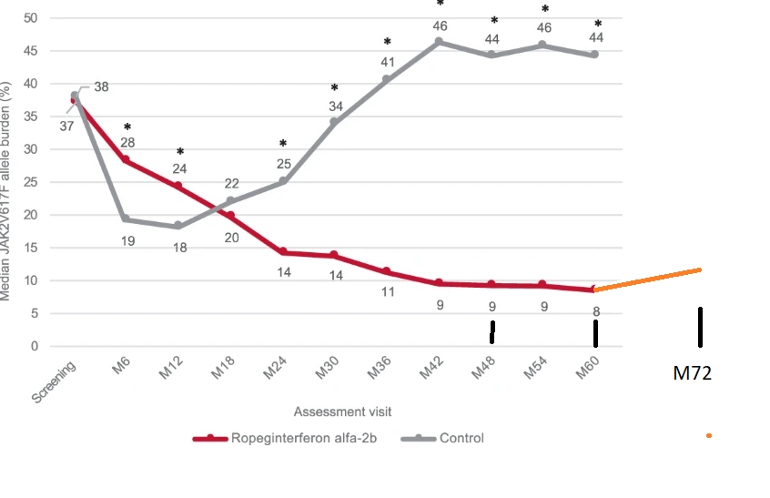
Top point: Data may show an increase in average allele after 5 years on Bes, see orange plot line.
--
I've been searching for 6 year update to the familiar plot here, it's not publicly available, and this may be why. The ASH hematology conference is next month and 6 year data is indirectly shown in a preview paper. Some summary papers are available, see links below.
In "Efficacy and Safety of Long-Term Ropeginterferon Alfa-2b Treatment in Patients with Low-Risk and High-Risk Polycythemia Vera (PV)" there is six year allele data. They divide it between 46pts Low Risk(LR) and 49 High Risk(HR). LR is under age 60 without history of thrombosis (clots in arteries or veins). Conveniently 46+49=95 pts which matches earlier docs for the 95 patients in the Conti-PV Ropeg study. So it's very likely the same patients and relevant to this plot.
The LR group was average VAF (allele burden) of 5.6%. HR group was 17.9%. I get a total average of 11.9VAF from that, as seen in this plot. See below how I calculated it.
This is not what we want to see, but I have been expecting it based on earlier PEG data, seen in old posts. Note that the Low risk group would be below this line at 5.6% while high risk is above it at 17.9. But there are no comparable numbers for prior years so we can use only the total average.
I believe a pattern will hold from other reports that those who get the lowest alleles are most likely to maintain it, but the Ropeg studies did not look there so far.
A separate note "LR patients were less likely to harbor additional non-driver mutations (11.4% vs 24.4%)." This may actually be the reverse, non-drivers contribute to risk rather than just incidental to it. This is discussed in detail in other posts including some non-drivers leading to less IFN response. They do continue the familiar relation between CHR (full blood response) and VAF, if indirectly.
--
There is a public number the authors have released in a different report, but it is not comparable to the average VAF plot here. "At six years, 20.7% of patients in the ropeginterferon alfa-2b group (n=19) achieved a JAK2V617F allele burden of less than 1%" But it does read nicely hence its easily found result.
ashpublications.org/ashclin...
--
We need that IFN + (something) therapy to get more patients in the best place. But we also still don't know how important it is to get to the lowest VAFs.
--
My simple calculation: 49ptsx17.9%= 877 (pts%). 46 x 5.6% = 257.
(877+257)/95= 11.9% average. The units work, but I have simplified it greatly. Any stats members are welcome to comment.
--
ASH links
The report discussed here: -ash.confex.com/ash/2022/web...
Ropeg related (not sure this link will work) - ash.confex.com/ash/2022/web...