There is a "discount" Rx company listing clear US pricing for Bes. This I believe works out to ~$170,000/year. If it goes 1dose/month after the 1st year, (for controlled HCT) it should be half that, ~84k/year.
But there is no "discount".
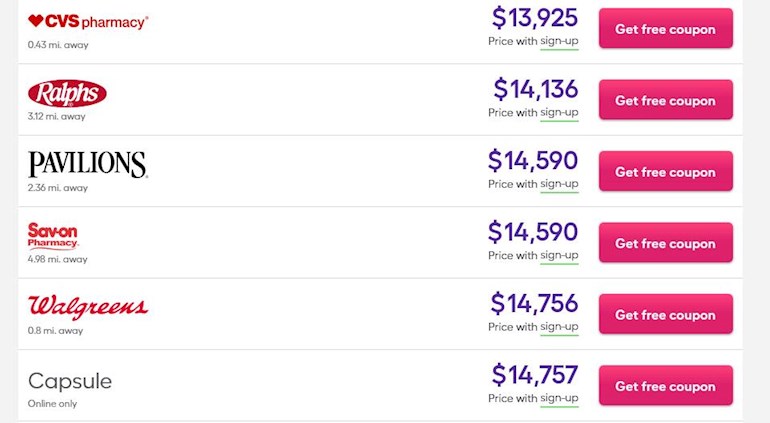
There is a "discount" Rx company listing clear US pricing for Bes. This I believe works out to ~$170,000/year. If it goes 1dose/month after the 1st year, (for controlled HCT) it should be half that, ~84k/year.
But there is no "discount".
Wow! I hope I can get my insurance to approve it! But I will still have to come up with the first $10,000 for my yearly deductible.
Cost for Pegasys is about $50,000/year only, quite high, but a lot lower compared to this one. WOW! $180,000/year, greedy merchants.
That's consistent with what we've seen others on PEG. I think PharmaEssentia looked carefully at the US system and decided they can get it.
I'm part wishing there is an insurance boycott for a short while to get them to a realistic price. A short sacrifice for a long term benefit. This just happened with Aduhelm, the new Alzheimer's medicine, with a 1/2 price cut.
As in this thread, Bes does have a real life competitor in PEG.
What exploitation of the US system!
This price tag of $180,000/year is unreasonably high for a drug that, in terms of effectiveness, flunked both of its Phase III randomized trials. FDA press announcement states that approval of Besremi was based on the observed efficacy solely from the single-arm Phase II PEGINVERA study!
Agree about the price, esp with PEG as an option. I also agree the FDA used Peginvera to a larger extent that one would expect. I recall reading that the longer studies (ContiPV) were also considered, but not as primary. Is there some additional info on these items? It is always helpful to know more.
healthunlocked.com/mpnvoice...
In this post I created plots from the text data from Conti PV. With respect to the parameters covered it is compelling in my opinion; the allele data is striking and the study is relatively easy to follow, unlike many others. There is also the less solid retrospective study on INF pointing to good things:
healthunlocked.com/mpnvoice...
There is also this post with some thoughts on Pegasys vs Ropeg:
healthunlocked.com/mpnvoice...
I have more recently been searching more reports on allele vs outcomes etc. I agree with the Silver MPN group that it is pointing in good directions. But large scale Ph 3 studies to better answer these questions will need to await the MITHRIDATE trial.
Interferon is a drug with delayed onset of action. It usually takes months, one year or longer to manifest its full drug effect. Therefore, it is counterintuitive to go from a weekly to a fortnightly dosing schedule, if the patient desires to have a quicker drug response to interferon.
The first of two Phase III trial for Besremi, ProudPV is a one-year study evaluating the drug vs HU, followed by a three-year Phase III ContiPV trial with the same returning study subjects.
However, for the treatment group only about two thirds of PV patients returning for ContiPV. There is clearly selection bias in this trial design, as those who responded to interferon treatment would enter ContiPV trials while the non-responders would likely not return and were screened off. The ContiPV results are apparently from the responders, not showing the whole picture of all PV patients.
There is a recent video with comments on Besremi from Dr. Richard Silver of Cornell, in which he proclaimed interferon of various forms, including Pegasys and Besremi, all have similar efficacy. The link is below:
vjhemonc.com/video/jtuu0vdx...
Agree INF acts much more slowly than HU for example.
For the weekly vs 14 day schedule, I think you refer to the dosing of Pegasys (PEG) vs Besremi (Bes) The key goal with all the pegalyted INFs is to keep the levels of active interferon more constant. Non-peg'd INF required ~3 doses per week with wide variations in INF levels in the body. This caused adverse events and tolerance problems. With the early Pegs dosing moved to 1/week with much improved tolerance. With Bes it's 1 or 2 per month with INF levels held the most constant and their claimed best tolerance. But I don't believe that dosing schedule has its main goal being absolute INF levels in the body, rather it is to keep a selected level more constant.
Regarding dose size, there are no detailed studies for PEG I'm aware of. We've seen widely different dosing on the Voice here 25mcg to ~well over 100.
For Bes there are detailed dosing instructions that are in fact similar as you suggest for increasing levels:
<<The recommended BESREMi starting dosage for patients not on hydroxyurea is 100 mcg by subcutaneous injection every two weeks....Increase the dose by 50 mcg every two weeks (up to a maximum of 500 mcg), until the hematological parameters are stabilized (hematocrit less than 45%, platelets less than 400 × 109/L, and leukocytes less than 10 × 109/L).>>
So the dose is increased until there is a response. But I'm not sure I would want to be dosed that way with an agent that is inherently slow acting. It's like flooring the gas pedal to get up to speed; before you can check the speedo you're going 90MPH. 90 leads to cops (adverse event). I'd rather push gently to give time to check my speed. The transition from HU might be a good compromise in which the starting dose is 50mcg, but still has that aggressive increase.
-----
In this paper they address exactly this question. They (and I) favor the gentle method:
(predates Bes approval)
ashpublications.org/hematol...
<<In addition, the relatively long time to response, with slow hematologic responses and subsequent deeper molecular responses, begs a philosophical dilemma between “hit it hard and hit it early” vs “slow and steady wins the race” since a linear and predictable dose-response and dose-toxicity relationship does not appear to exist, and maintaining patients on a tolerable dose for a long period of time may be of particular importance. Our approach is to start at a low dose, 45 µg weekly, of pegIFN alpha-2a (Pegasys, the only currently available US formulation) for a minimum of 4 weeks. Close monitoring of mood, liver, and thyroid function dictate further dose modifications or interruptions. Some patients stay at 45 µg weekly, and others increase to 90 µg weekly; doses as high as 135 or 180 µg weekly are less common but can also be tolerated by some and may be helpful in the absence of a hematologic response at lower doses.>>
------
Regarding the ContiPV study, according to:
mpn-hub.com/medical-informa...
<<89.6% of patients (n = 95) treated with ropeginterferon alfa-2b in the PROUD-PV study entered in the CONTINUATION-PV extension stud>>
So that is ~90% INF entering the long term study. 2/3 does show up for HU <<68.5% of patients (n = 76) treated with hydroxyurea in the PROUD-PV study enrolled in the CONTINUATION-PV extension>> This suggests Bes was more worth sticking with.
Agree with the video that INFs are all similar, it's how they are released into the body that is most different. I have read of some diffs between the alpha (a) and alpha (b) INFs. (a) is more selective to the blood and one other area I don't recall, (b) is more widely distributed in the body. (I don't have this study handy, could look for it if desired) PEG uses alpha (a) I believe, Bes uses alpha (b). Why Bes chose (b) is an interesting question.
Thank you for the info. You're very knowledgeable about interferon. It is a fickle drug, by that I mean the drug has different effects on different patients. Hence, there is not an exact amount that one should take. Everyone reacts to the drug differently.
There was some video in which Ruben Mesa was promoting interferon to his fellow panelists, one of which claimed to treat his PV patient for some 20 years. This panelist went on to say that he had to adjust dose amounts up and down several times over the years and that the PV patient using interferon should find a Dr who can outlive the patient.
In comparison with HU, interferon is more costly. You know, insurance companies used to reject claims using Pegasys to treat PV, on the basis of its being off-label. With the dynamite price of $180,000 by Besremi, Genentech's product looks to be much friendlier.
The prices of medical care in the US is astronomical!! I faint, just looking at this price list, even when it’s halved! I’m in Vienna, Austria.
The cost is a few thousand dollars more per year than Jakafi, so there is precedent. At this cost point, there is going to be a lot of resistance to pay for it. On the bright side, perhaps this will make it easier for people to access Pegasys.
I am doing well on Pegasys and Medicare covers most of it. Personally, I see little benefit in switching to Besremi at this price even if Medicare pays most of the cost.
Hi
I thought you might find this interesting it’s summary of all things Besremi submitted to federal authorities in Germany. It gives costs etc as well as summaries of studies.
Currently it’s only available to PV patients here in Germany and the vast majority unless intolerant seem to be on it.
It’s in English
g-ba.de/downloads/40-1465-6...
Thanks for the info. Only through the forum would such an item be available to us.
With this underwhelming report it's surprising Bes was approved for anything. Any INF does not look very good at 12 months.
<<Overall, the G-BA finds that the described methodological deficiencies of the
CONTINATION-PV study make interpretation of its finding s sufficiently unreliable to support a claim of additional benefit. Consequently, only the data from the PROUD -PV study, covering an observation period of 12 months, have been considered in the present benefit assessment>>
But one reason for the rejection of the ContiPV arm was <,Of these, 95 patients switched from the ropeginterferon- alfa-2b arm (approx. 75%) >> while in
mpn-hub.com/medical-informa...
<<89.6% of patients (n = 95) treated with ropeginterferon alfa-2b in the PROUD-PV study entered in the CONTINUATION-PV extension study>>
89.6 initially switching is much better than 75%. Something missing.
And <<a significantly higher percentage of patients in the hydroxyurea arm of the PROUD-PV study were found to have been hypertensive at the time of screening of the PROUD-PV study (56.6% vs 34.3%), to have received phlebotomies (42.1% vs 22.9%), or to have a higher
median haematocrit (49.9% vs 46%)>>
This in my opinion just suggests that HU wasn't working that well in the Proud arm. I'm sure some real experts can explain these better.
This one however is a good point:
<<For example, in the (contiPV) study only patients were enrolled who had benefited
from ropeginterferon alfa-2b as evidenced by normalisation or reduction of relevant blood
levels (haematocrit, leucocytes, thrombocytes), normalisation of spleen size or any other
clear medical benefit (such as normalisation of disease-associated microvascular symptoms
or relevant reduction of JAK-2 allelic load)>>
But how about the HU arm, did they also admit only HU responders?
The main problem is the study (and most others) was not designed to consider molecular responses since this benefit is not official enough yet. These plots I've posted many times are so extreme on this that any bias on this parameter I think is wiped out. But without allele as a parameter the experts can't talk about it. The hematological responses in the center plot are indeed less striking, although fine by me, but biases could be a bigger issue there.
Interesting the US FDA approval was based on only PEINVERA for efficacy but ContiPV was included for safety.
drugs.com/newdrugs/fda-appr...
Distasteful price, in my opinion. Spinning gold on ill people's needs !! Sometimes I would like to do like Jan Palach did to show protest. But I don't like fire :/
I cannot stop thinking about the fact that our "diseases" aren't real diseases, but dysfunctions. There must be other ways !!
What does poor Americans do ???
I see in your prior posts you are or were on PEG. Are you still? Is it still working ok?
I agree about the price. The company has a program to offer at low or zero cost for uninsured and the like. But you're right the US system is too complicated and the company takes advantage of that.
Regarding the name for our conditions, it was classified as "Cancer" ~15 years ago and that triggered much of the research and money that is going to MPN in recent years. Strange how that works.
Yes, strangely strange, but ugly normal. And yes , a good thing that they finally agreed about the cancer thing. It has an energizing effect on scientists and their feeders.
I'm glad to hear that you have certain rules for the ones of "less luck".
I'm still on Pegasys ( 65gm fortnightly) In my own opinion it works well enough. I'm getting slightly zombie'ish during wintertime, so vitamins surely has something to say.
There is a German mineral complex named "Basica" (Protina Pharm. GmbH) which really
helps. Quite natural, when we clean out much good stuff by our injections.