In case you missed GO2 Foundation's weekly update last week about COVID-19, I will copy the text here and include the link to our webpage that is full of additional information to keep you informed.
Webpage: go2foundation.org/blog/upda...
The first case of COVID-19 in the USA was reported on 1/20/2020—over 10 months ago. Since then, the country has reported 15,718,811cases and 294,535 deaths as of December 12 (per the Centers Disease Control and Prevention). With 80% of US counties reporting more travel than last year over Thanksgiving weekend in November 2020 despite warnings from the CDC, we are finally seeing the impact of this holiday surge.
The number of new cases is up more than 20 percent from 2 weeks ago
The number of hospitalizations has increased by 21 percent
The number of deaths has jumped 39 percent, with the United States surpassing 3,000 deaths in 1 day for the first time
On December 11, the United States Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the first SARS-CoV-2 mRNA vaccine, BNT162b2, manufactured by the pharmaceutical giant, Pfizer. For a description of how mRNA vaccines work, please check the website link above and follow the links.
The New York times reported that large-scale manufacturing and distribution of vaccines has already begun, with the first dosing to start on December 14, 2020. This huge milestone is a positive step towards fighting the COVID-19 pandemic. However, it is important to keep in mind that it will take a considerable amount of time before the entire US population is either vaccinated or immune to COVID-19 through natural infection. With the year-end holidays around the corner and an anticipated increase in travel, the CDC has extended its travel advisory to include the winter break. We encourage our community members to weigh the risks and benefits of travel during this winter. Thanks to the vaccine, the end of the pandemic may be on the horizon. Till such time, maintaining public health measures such as masking, handwashing, social distancing, and minimizing non-essential travel are our best bets for protection.
How was the Pfizer vaccine approved?
The vaccine was approved based on a randomized, double-blind Phase 2/3 clinical trial. A total of 43,548 participants (older than 16 years) received either two doses of the vaccine or a placebo injection three weeks apart. Participants were followed for safety and for the development of symptomatic COVID-19 for approximately 2 months. Eight participants in the vaccine group developed symptomatic COVID-19, whereas 162 participants in the placebo group developed symptomatic COVID-19. The vaccine was found to be 95% effective in preventing severe COVID-19 symptoms i.e., for every 100 people who received the vaccine, 95 were protected from developing severe COVID-19.
Is the Pfizer vaccine safe?
Side effects reported by trial participants were generally mild or moderate, and reactions were less common and milder in older adults than in younger adults. Those who received the vaccine had localized reactions at the injection site (pain, redness, swelling) and systemic reactions (e.g., fever, headache, muscle ache) at higher rates than placebo recipients, with more reactions following the second dose. Severe fatigue was observed in approximately 4% of vaccine recipients. However, this rate of severe fatigue is also lower than that observed in recipients of approved influenza vaccines for older adults. Serious side effects were similar in both the vaccine and placebo groups (0.6% and 0.5%, respectively).
It is important to keep in mind that we do not have long-term follow-up data from this clinical trial. Sometimes, side effects may show up after months of follow-up. Also, vaccination began in the United Kingdom last week. Two individuals with a history of severe allergic reactions were reported to have had a severe reaction to the vaccine. These individuals carried an EpiPen and use of the pen was sufficient to counteract the allergic reaction. It is anticipated that these reactions will be very rare given that such safety issues were not seen in the large clinical trial. The public health benefits of distributing this vaccine still far outweigh any perceived risks.
What is not known about the Pfizer vaccine?
We do not know whether the vaccine will be effective for more than 2 months, because participants have only been followed for 2 months so far. However, additional data continues to be gathered.
Children (less than 16 years of age), pregnant women, and immunocompromised patients (such as those who have received cell-based therapies or chemotherapy for their cancer) were not included in the study. We do not know if the virus will be safe (in children and pregnant women) or effective (in immunocompromised patients who may not mount an immune response) in the groups excluded from the clinical trial.
The vaccine involves two doses given three weeks apart. The first dose “primes” the immune system to respond while the second dose “boosts” that response. If someone misses the second dose, we do not know whether the vaccine will still be effective.
We don’t yet know whether the vaccine will prevent the recipient from getting infected or from spreading COVID-19. Again, we need more data. We’ll need to continue practicing public health measures such as masking and social distancing even after receiving the vaccine, at least in the near term.
When will I receive the vaccine?
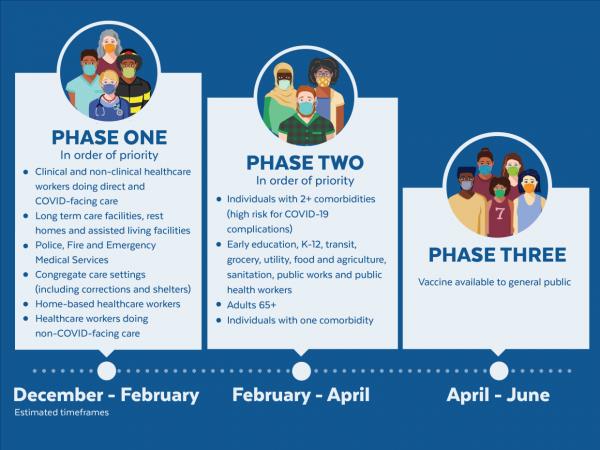
The United States is adopting a phased approach to roll out large-scale vaccination. The phased approach prioritizes the most essential and the most vulnerable of our population as the first recipients of the vaccine, given the initial limited supply of vaccines. The following figure shows how the state of Massachusetts will use the phased approach for distributing vaccines. It is anticipated that patients with lung cancer will receive vaccines in Phase 1 or 2.
As of December 2020, the Advisory Committee on Immunization Practices (ACIP) recommended that both 1) health care personnel and 2) residents of long-term care facilities be offered COVID-19 vaccine in the initial phase of the vaccination program (Phase 1a).
Each state in the United States is likely to have specific vaccination guidelines tailored to their own specific needs. For information specific to your state, please check the links on the webpage noted at the top of this post.
An important population for our community is caregivers to patients with lung cancer. If you are the primary caregiver for your loved one, please check your eligibility for receiving the vaccine.
This will be our last update of the year. We wish everyone a safe and peaceful Holiday Season! Please continue to maintain social distancing, wash hands, mask, and minimize non-essential travel. See you in 2021!
Resources and websites
IASLC’s Guide to COVID-19 and Lung Cancer
The National Cancer Institute website for COVID-19 and emergency preparedness COVID-19: What People with Cancer Should Know
Updates from the World Health Organization (WHO) and the US Centers for Disease Control and Prevention (CDC)
Johns Hopkins COVID-19 Resource Center
Interactive map of US COVID-19 cases by state
COVID-19 in patients with cancer: managing a pandemic within a pandemic
You can find information specific to your state or city or town on your health department’s website.
Directory of state department of health websites
Directory of local health department websites
American Medical Association resources for healthcare providers.