A guide to Fall vaccine options - by KATELYN JETELINA AND CAITLIN RIVERS AUG 17
yourlocalepidemiologist.sub...
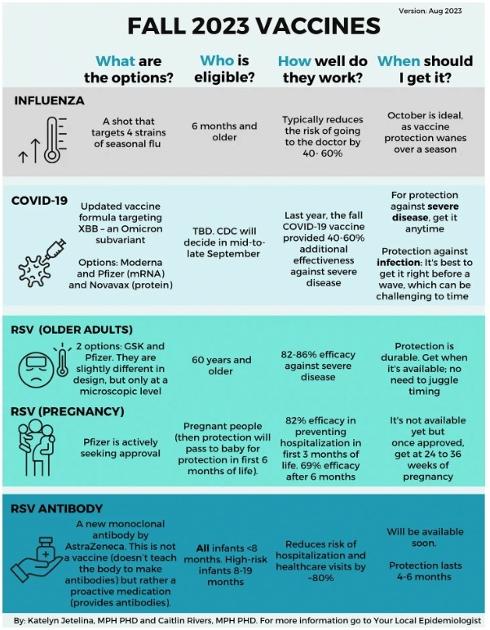
It’s the first time we have vaccines for all three fall respiratory viruses that hospitalize and kill hundreds of thousands annually. This is a big deal, that is, if we utilize them. So, we thought collecting information about them in one place would be helpful. This is the what, who, and when for each.
At the bottom, we include a one-page PDF summary. (Many physician offices and health departments have previously found it helpful to print these.)
Seasonal influenza (flu)
What: The vaccine covers four strains of seasonal flu offered by four pharmaceutical companies. Selecting vaccine strains for rapidly changing viruses, like flu and COVID-19, is both an art and a science, so the vaccine formula doesn’t always align perfectly with the circulating virus. We are optimistic that this year’s is a good match because the vaccine composition matches flu strains that recently circulated in Australia (which is a good predictor of the upcoming Northern Hemisphere season). During the years when the vaccine does match, it can reduce the risk of needing to go to the doctor by 40% to 60%.
Who: Everyone ages 6 months and older. There are special formulations to provide added protection to older adults. Children should get two shots during their first flu season.
When: Protection wanes throughout a season. For most people, October is the best time to get vaccinated so that you’re fully protected before a flu wave. The complete list of timing recommendations for specific populations (pregnant people, older adults, young children) is available here.
COVID-19 vaccine
What: The fall Covid-19 vaccine has an updated formula targeting XBB.1.5, which should be a good match to the currently circulating Omicron subvariant. Moderna, Pfizer, and Novavax all plan to have boosters on the market this fall.
Who: We don’t know yet but should know in mid-to-late September. Why the delay? The CDC only determines who is eligible after the FDA fully approves the vaccine. The FDA can only fully approve it once the pharma companies submit data showing the vaccines are safe after manufacturing. We are waiting for this process to play out.
Last year, though, eligibility was dependent on the manufacturer, and it will likely be the same this year:
• Moderna: 6 months and older
• Pfizer: 6 months and older
• Novavax: 18 years and older
When: Guidance will be provided by the CDC soon. (Hopefully, they will guide recently infected people, too.) For protection against severe disease, you can get the vaccine when it becomes available because this kind of protection lasts longer. For protection against infection, though, keep in mind that protection wanes in a few months, so it’s best to get vaccinated right before a wave. Of course, this can be challenging to time.
More info: To understand why we need an updated vaccine and what clinical trials found, go to this previous YLE post.
RSV vaccine for older adults
What: For the first time, an RSV vaccine is available and from two manufacturers— GSK and Pfizer. Both effectively protect against severe illness, with 82-86% efficacy. The two vaccines are slightly different in design, but only at a microscopic level. And side effects like fever and body aches are uncommon.
Who: People ages 60 and older “may” get the vaccine in the U.S. In the U.K., those over 75 years “should” get the vaccine. People with underlying health conditions (like heart or lung disease or diabetes) and those living in long-term care facilities should strongly consider the vaccine.
When: They are available now. RSV vaccines do not wane as quickly as flu and COVID-19 vaccines, so getting one now should protect you throughout the entire season (and maybe even next season).
More info: For a breakdown of the clinical trial findings, go to this previous YLE post.
RSV vaccine for pregnancy
What: Pfizer is actively pursuing approvals for an RSV vaccine given to pregnant people. The protection will pass from mother to baby so that the baby is protected in the first 6 months of life, which is the riskiest time for severe RSV. Clinical trials showed 82% efficacy in preventing hospitalization during the first 3 months of life, and 69% efficacy at 6 months.
Who: If approved, the vaccine would be given between 24 to 36 weeks of pregnancy.
When: This vaccine is not yet available. It is still going through review by the FDA and CDC. We may have a decision this month. If so, it may become available this fall, but the timing is uncertain.
RSV medication for infants
What: AstraZeneca has a new monoclonal antibody called Beyfortus, which protects against severe RSV in infants. This is not a vaccine (i.e., doesn’t teach the body to make antibodies) but rather a medication (it provides antibodies). In clinical trials, it reduced the risk of hospitalization and healthcare visits by ~80%.
Who: All infants under 8 months should get it for their first RSV season. High-risk children between 8 months to 19 months should also get it. High-risk categories include:
• Chronic lung disease of prematurity
• Severe immunocompromise
• Cystic fibrosis
• American Indian and Alaska Native children
When: Beyfortus is not available yet, but the manufacturer has committed to making it available for this RSV season. The protection lasts about 4-6 months, so get this as soon as it’s available.
Bottom line
Get protected! It is one of the best things you can do this fall and winter to stay healthy and minimize disruption.
Love, YLE and CR
P.S. We know many people have many questions. Please comment or send them to us, as we plan on following up with a FAQ. And, of course, it’s always a good idea to consult with your pharmacy or clinician.
P.S.S. Below is a PDF version (English and Spanish) of the summary for paid subscribers. Feel free to download, print, share, or doodle on. ...
Subscribe to Your Local Epidemiologist to read the rest.
© 2023 Your Local Epidemiologist
548 Market Street PMB 72296, San Francisco, CA 94104