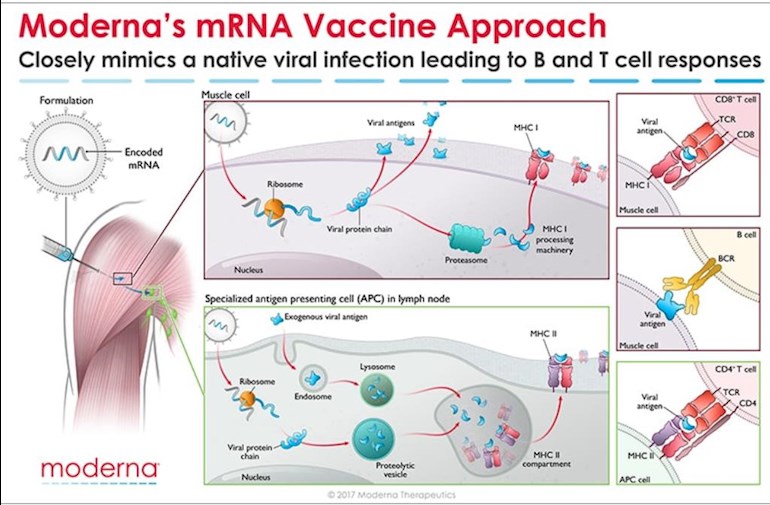
This is the best diagram I've seen so far depicting the way Moderna's mRNA 1273 vaccine is expected to work.* It highlights the fact that the introduced mRNA (spike protein mRNA1273 in the case of the SARS-CoV2 vaccine), enters myocytes in muscle tissue as well as macrophages, dendritic and B cells, in lymph tissue, where it is translated through the process of protein synthesis, producing the SARS-CoV2 spike protein antigen, which is subsequently either secreted from the cells, or processed and presented at the cell surface,
1. available for binding to the BCR (B cell receptor) of a nascent B cell leading to its conversion to an anti-spike protein antibody producing plasma cell, and perhaps a memory cell B cell as well,
2. or binding to a T cell receptor leading to activation of the T cell.
Reading Neil's recent post about the importance of T cells in fighting COVID-19 led me to post this diagram showing how Moderna's vaccine is not only expected to lead ultimately to the synthesis by B-cells of anti-spike protein antibodies, but that it also is expected to activate T-cells as shown in the diagram.
modernatx.com/moderna-blog/...
* The cartoon was made in 2017 when Moderna was studying other mRNA vaccines.
Thanks Neil for the nudge I needed to present the Moderna approach diagram which I just discovered this evening.
gardening-girl
Some other reading of possible interest:
1. Links to 2017 papers from Moderna explaining their vaccine strategy which includes modifying ribonucleotide bases in the RNA to make it more stable, and encapsulating the RNA into a specially designed lipid nanoparticle:
cell.com/molecular-therapy-...
sciencedirect.com/science/a...
2. The story of mRNA: How a once-dismissed idea became a leading technology in the Covid vaccine race
statnews.com/2020/11/10/the...
3. Why does Pfizer's COVID-19 vaccine need to be kept at a lower temperature than Moderna's: