ANZUP Mini ASM 2020: The Global Context of the TheraP Trial and the Pathway to Approval. Prospective randomized trial.
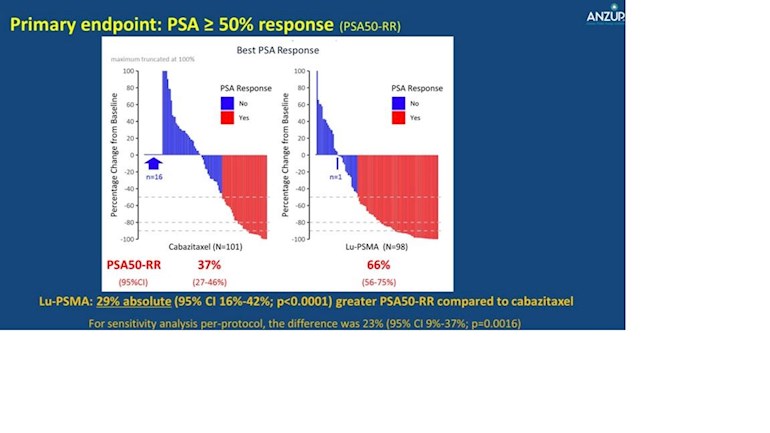
More patients had a =/>50% decrease in the PSA when treated with Lu 177 PSMA than patients treated with Cabazitaxel (Jevtana)..
ANZUP Mini ASM 2020: The Global Context of the TheraP Trial and the Pathway to Approval. Prospective randomized trial.
More patients had a =/>50% decrease in the PSA when treated with Lu 177 PSMA than patients treated with Cabazitaxel (Jevtana)..
I posted some questions that remain unanswered here:
The patients in the Cabazitaxel group were not checked if ineligible.
I did not understand, they were not checked for what? The interesting thing to me, was that in this population Lu 177 PSMA was more effective than Jevtana and that only 37% had a 50% or more decrease in PSA when treated with Jevtana.
First of all, I would choose the Lu177 treatment over the Cabazitaxel therapy simply because it has fewer side effects and, according to this study, is more efficient than Cabazitaxel.
However, I think Dr. Hofman should not have excluded these patients: "...there could not be sites of disease which were FDG positive but PSMA negative". I think these patients should at least get a chance. Thang reported the poor outcomes of these patients after being excluded and concludes, one does not know if Lu177 would have helped them.
We know that Cabacitaxel will not work for a certain percentage of patients but we do not have a way to know who these are in advance. Dr. Hofman excludes the patients where Lu177 is likely not to work. So I think there is a bias here.
euoncology.europeanurology....
I agree, that they selected the patients and the results apply only to the selected population.
There is not bias in the study since the patients that were selected with Ga 68 PSMA and FDG PET/CT studies were randomized to Lu 177 PSMA or Cabazitaxel.
There is the unknown of the response to Cabazitaxel or Lu 177 PSMA of the patients that were excluded.
Ok, I did not observe that also the Cabazitaxel group could not be FDG positive and PSMA negative. Maybe the headline should be: Lu177 more effective than Cabazitaxel in a selected patient group.
Still I think the excluded patients should be offered Lu177 and not left alone.
Perhaps they did treated them, but it is not included in this study.
I believe the excluded patients have some form of treatment induced neuroendocrine cancer since a large proportion of their cancers did not express PSMA, It is expected that in this population the response to Lu 177 PSMA and Jevtana may be low .
Tango, I provided a link to the results: "..died, with a median OS of 2.5 mo". I would have tried to help them, at least that's what I feel.
Sorry , I did not have time to read your link before.
They did helped them. The treatments the excluded patients received could be found in appendix A in this link:
sciencedirect.com/science/a...
I think they found a way to identify a population of patients who will not respond or respond poorly to Cabazitaxel and/or other therapies by using a combination of Ga 68 PSMA and FDG PET/CT scans. I believe this is pretty remarkable.
Thanks for the engaging discussion. I agree that these patients could have been disqualified from the trial but still offered treatment, and we could have gained knowledge from them. In the appendix, Patient 1 could have had 275ml of tumor debulked with 177Lu-PSMA. If the PSMA avid disease was a proper subset of the FDG avid disease (408ml), then most of the disease could have been treated in this case. Or Patient 16, where there is much more PSMA avidity than FDG avidity, even greater debulking could have been successful. (I admit that I did not read the paper to see how they define the percentage of discordance but at 17% for Patient 16 it seems low).
My understanding is that there could be a competition for nutrients and other factors among different cells in the environment of the metastases.
There is the possibility that by debulking the low expressing PSMA cells, the FDG positive cells could have a faster progression and cause the cancer to progress more rapidly.
I recall one of Hofman's slides is a photo of a bunch of military shells exploding all at once suggesting that if the beta particle doesn't kill the cell that it got absorbed into, it is likely to hit *something* in the scope of treatment. And I would think that those could be FDG+ cells as easily as PSMA+ cells.
One should remember the cells we are talking about have a low PSMA expression. No too many "bombs" will be coming their way.
You mean this image:
Tango, here is another study in which they looked into discordant lesions detected by PSMA PET/CT and FDG PET/CT.
link.springer.com/article/1...
In this study they treated the patients with mainly FDG positive lesions and PSMA negative lesions with Lu177 as well. These had an overall survival of six months while the patients in the study by Thang had an overall survival of 2.5 months only. The discordant subgroup in the study by Thang had an overall survival of 3.9 months. So treating these discordant patients with Lu177 is beneficial.
It's not unlikely to work. It won't work. The LU 177 is attached to a ligand that attaches to the PSMA receptor. If there's no PSMA expression , there's nothing for the Lu 177 to attach to. So from a trial perspective there is no point in including them. it would add no useful data and cost a fortune.
It would be like testing an antibiotic on a infection known to be viral.
Patients usually have a mix of PSMA positive and negative tumor. Of cause, if you have PSMA negative tumor only, there is no point in treating this patient. But if you can remove about 40% of the tumor, it could be beneficial. Especially when the visceral mets are positive.
Thang writes: Study limitations include uncertainty for imaging thresholds that define low PSMA expression. It is also possible that theranostic therapy could have improved survival in this cohort.
sciencedirect.com/science/a...
It's true it might (that's also a big might) have some benefit for the patient but it would make the trial data not useful. and remember this is a very expensive treatment, so quite reasonably the researchers need clean data to identify if the treatment has benefit.There's certainly an argument that people who don't qualify could get the drug anyway, and just be excluded from the trial data, but then there is the question of who is going to pay for it.