At last. Although is is dated 10 April 2014, I have only just found this document published by Amdipharam about the differences between Eltroxin and their generic levothyroxine - and how these differences will be disappearing.
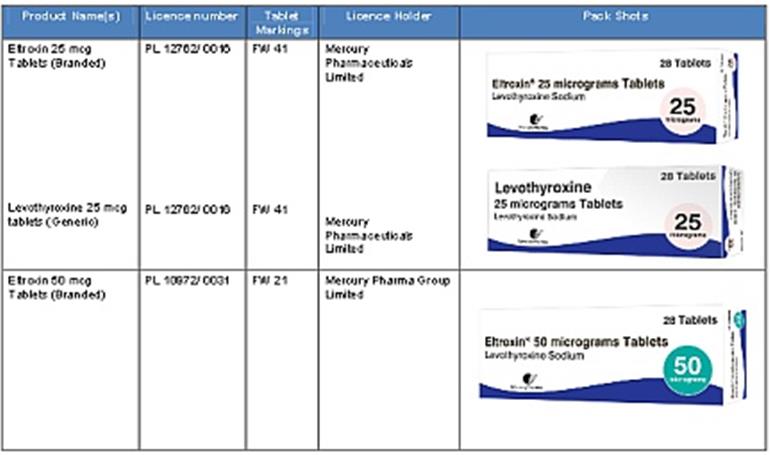
Levothyroxine and Eltroxin tablets from Amdipharm Mercury (formerly Mercury Pharma) (“AMCo”)
We understand that there is confusion for some patients about which products are available, and whether products marketed by AMCo as branded Eltroxin or as generic levothyroxine are the same or different.
This communication is provided to try and help to clear any confusion.
amcolimited.com/media/21282...
Rod