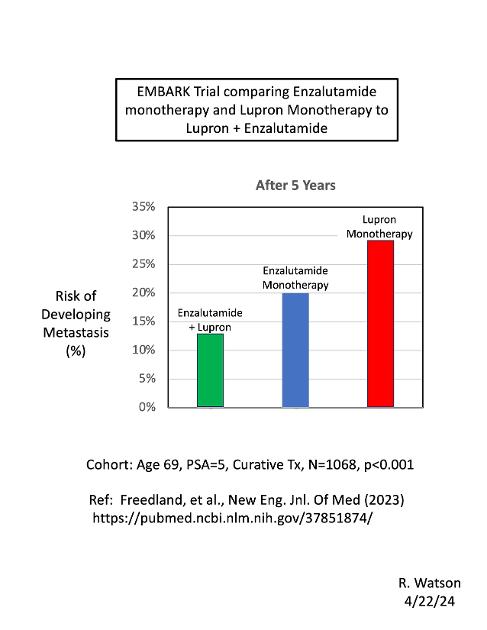
This chart compares the risk of developing metastasis from prostate cancer, after 5 years from curative treatment, between three different arms of the EMBARK study (published in 2023):
(1) Lupron monotherapy;
(2) Enzalutamide monotherapy; and
(3) Enzalutamide + Lupron combination therapy.
The cohort was men with PCa, average age = 69, PSA = 5, after curative treatment (RP or RT).
This chart shows significant differences between the three arms. Lupron monotherapy had the greatest risk of developing metastasis after 5 years (a 28.6 %), compared to a 20.0% risk for Enzalutamide monotherapy. The combination of Enzalutamide + Lupron had the lowest risk (12.7%). p < 0.001 (N=1068).
Combinations of Lupron (or Orgovx, etc.) with other 2nd-generation Anti-Androgens (ARSIs), such as darolutamide and apalutamide, might give similar results.