Possible simple dietary intervention to increase autophagy in PCa and boost efficacy of ARSIs.
Abstract & Intro (+ Section snippets in ScienceDirect link):
* * *
Abstract
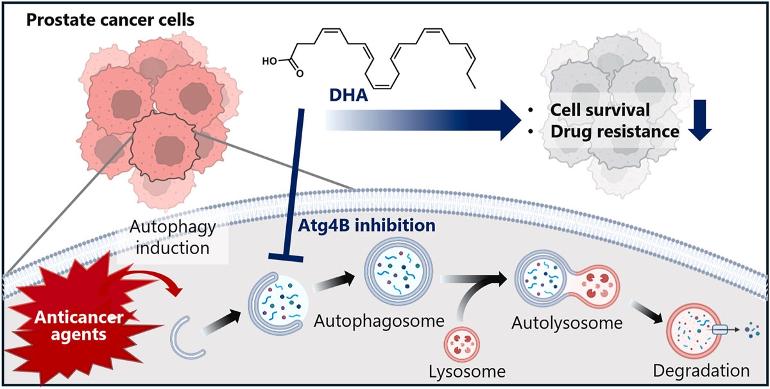
Autophagy induction in cancer is involved in cancer progression and the acquisition of resistance to anticancer agents. Therefore, autophagy is considered a potential therapeutic target in cancer therapy. In this study, we found that long-chain fatty acids (LCFAs) have inhibitory effects on Atg4B, which is essential for autophagosome formation, through screening based on the pharmacophore of 21f, a recently developed Atg4B inhibitor. Among these fatty acids, docosahexaenoic acid (DHA), a polyunsaturated fatty acid, exhibited the most potent Atg4B inhibitory activity. DHA inhibited autophagy induced by androgen receptor signaling inhibitors (ARSI) in LNCaP and 22Rv1 prostate cancer cells and significantly increased apoptotic cell death. Furthermore, we investigated the effect of DHA on resistance to ARSI by establishing darolutamide-resistant prostate cancer 22Rv1 (22Rv1/Dar) cells, which had developed resistance to darolutamide, a novel ARSI. At baseline, 22Rv1/Dar cells showed a higher autophagy level than parental 22Rv1 cells. DHA significantly suppressed Dar-induced autophagy and sensitized 22Rv1/Dar cells by inducing apoptotic cell death through mitochondrial dysfunction. These results suggest that DHA supplementation may improve prostate cancer therapy with ARSI.
Introduction
The number of prostate cancer patients is rapidly increasing worldwide. In 2020, more than 1.4 million people were newly diagnosed with prostate cancer, making it the second most common cancer in men after lung cancer. Prostate cancer accounts for approximately 400,000 deaths and ranks as the fifth leading cause of cancer-related deaths [1]. Most prostate cancers depend on androgens for growth [2,3], so androgen deprivation therapy (ADT) is a primary treatment, with a 5-year survival rate exceeding 99 % when treated in the early stages. However, in metastatic cancer cases, the survival rate drops to 30 %. Additionally, ADT is highly effective in the early stages of treatment, but acquired resistance occurs within approximately 18–36 months, leading to castration-resistant prostate cancer (CRPC) [4]. Androgen receptor signaling inhibitors (ARSI), including a cytochrome P450 17A1 inhibitor (abiraterone [Abi]) and androgen receptor antagonists (enzalutamide [Enz], apalutamide, and darolutamide [Dar]) or microtubule inhibitors (docetaxel, cabazitaxel [Cab]) are used for CRPC treatment. These ARSIs are effective initially, but resistance is eventually acquired in most cases [[5], [6], [7]]. Prostate cancer cells induce autophagy in response to androgen removal and ARSIs [8]. Autophagy contributes to the maintenance of cellular homeostasis by sequestering misfolded or mutated proteins and damaged or aging organelles into double-membrane vesicles called autophagosomes, which subsequently fuse with lysosomes for degradation of their contents [9]. Induction of autophagy in cancer cells is implicated in malignant progression and acquisition of resistance to anticancer drugs [10,11]. In prostate cancer, autophagy induction contributes to acquired resistance to ARSI. Therefore, targeting autophagy is therapeutically promising for overcoming resistance [[12], [13], [14], [15]].
Hydroxychloroquine (HCQ), an autophagy inhibitor that acts by inhibiting lysosomes, has shown efficacy in various cancers, including metastatic pancreatic cancer [16], non-small cell lung cancer [17], refractory multiple myeloma [18], and malignant melanoma [19]. However, autophagy inhibitors approved by the U.S. Food and Drug Administration or equivalent organizations in other countries, including Japan, have yet to be developed. Autophagosome formation involves two ubiquitin-like modification pathways: autophagy-related (Atg) 5-Atg12-Atg16L and microtubule-associated protein 1 light chain 3 (LC3) precursor (pro-LC3), mediated by the E1-like enzyme Atg7. Pro-LC3 is cleaved at the C-terminal 22 residues by Atg4 to form LC3-I, which binds to phosphatidylethanolamine (PE, a phospholipid molecule) to form LC3-II. LC3-II is then recruited to the autophagosome membrane, where it is expressed on both inner and outer surfaces [20,21]. Upon autophagosome maturation, Atg4 mediates the detachment of PE from LC3-II on the outer membrane. Subsequently, fusion between the autophagosome and lysosome membranes forms an autolysosome, where the enclosed contents are degraded. Among the four homologs of Atg4 (Atg4A, Atg4B, Atg4C, and Atg4D), Atg4B exhibits the highest activity against LC3 processing in humans [22]. Knockdown of Atg4B [23,24] and transfection of inactive mutant Atg4B C74A [25] have been reported to inhibit autophagy.
In a previous study, we discovered Atg4B inhibitor 17 and developed 21f, a potent competitive inhibitor against Atg4B through structure-based optimization [26]. We also reported that 21f enhances the anticancer activity of ARSIs by inhibiting autophagy in prostate cancer cells. Natural compounds with Atg4B inhibitory activity have not been reported. In this study, we searched for natural compounds with Atg4B inhibitory activity based on the structural features of the developed 21f and found that long-chain fatty acids (LCFAs) possess Atg4B inhibitory activity. Among them, docosahexaenoic acid (DHA) strongly inhibits Atg4B at low concentrations. Furthermore, treatment with low DHA concentrations inhibits ARSI-induced autophagy in prostate cancer cells, significantly enhancing the anticancer activity. (All emphasis added.)
* * *
Along with the above, the Section snippets can be found here:
Docosahexaenoic acid enhances the treatment efficacy for castration-resistant prostate cancer by inhibiting autophagy through Atg4B inhibition,
Archive of Biochemistry & Biophysics, 2024 Aug 23:760:110135. Online ahead of print.
sciencedirect.com/science/a...
DHA (+ EPA) is also essential for cognitive heath, so a potential two-bagger for those on an ARSI?
Stay S&W,
Ciao - cujoe